| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website https://www.neurores.org |
Original Article
Volume 11, Number 1-2, April 2021, pages 14-19
Apnea Test Safety in Brain Death: A Single-Center Retrospective Cohort Analysis
Talita Sansonia, e, Nicholas Nascimentoa, Gabriel Francob, Rui Morenoc, Alexandre Barrosa, Venancio Filhod, Helder Zambellid, Ana Paula Gasparottoa, Luiz Antonio Sardinhad, Antonio Luis Falcaoa
aIntensive Care Unit, Discipline of Physiology and Surgical Metabology, Department of Surgery, Faculty of Medical Sciences, State University of Campinas (Unicamp), Tessalia Viera de Camargo St. 126, University Town Zeferino Vaz, Campinas, Sao Paulo 13083-887, Brazil
bDepartment of Statistics, Instituto de Matematica, Estatistica e Computacao Cientifica da Universidade Estadual de Campinas (UNICAMP), R. Sergio Buarque de Holanda, 651 - Cidade Universitaria, Campinas-SP 13083-859, Brazil
cNeurocritical and Trauma Intensive Care Unit, Hospital de Sao Jose, Centro Hospitalar Universitario de Lisboa Central, Lisbon, Portugal
dTransplant Organ Search Organization, Hospital das Clinicas, Universidade Estadual de Campinas (UNICAMP), Rua Tessalia Vieira de Camargo 126, Cidade Universitaria Zeferino Vaz, Campinas, Sao Paulo 13083-887, Brazil
eCorresponding Author: Talita Sansoni, Intensive Care Unit, Discipline of Physiology and Surgical Metabology, Department of Surgery, Faculty of Medical Sciences, State University of Campinas (Unicamp), Tessalia Viera de Camargo St. 126, University Town Zeferino Vaz, Campinas, Sao Paulo 13083-887, Brazil
Manuscript submitted November 4, 2020, accepted December 24, 2020, published online February 8, 2021
Short title: Apnea Test Safety in Brain Death
doi: https://doi.org/10.14740/jnr646
| Abstract | ▴Top |
Background: The apnea test, which is considered positive when no spontaneous breathing movements are observed following maximal hypercapnia (PaCO2 > 55 mm Hg) respiratory center stimulation, was critically evaluated in this study by assessment of blood gas analyses performed during brain death protocols from 2010 to 2017, in the intensive care units of the Universidade Estadual de Campinas (UNICAMP).
Methods: A retrospective cohort analysis based on the intensive care unit and Transplant Organ Search Organization data banks. Blood gas analyses before (pre-first and -second apnea tests) as after (after-first and -second apnea tests) were assessed. Descriptive statistical analyses of the numerical variables (such as pH, PaO2, PaCO2, HCO3, SatO2) with mean values and standard deviation, medians, and quartiles were performed. The Student’s t-test was used for pairwise group comparisons. A P < 0.05 level was adopted for significance.
Results: Eighty-seven protocols were evaluated. The mean apnea test duration was 11 min. All of the patients were under vasoactive drugs. Only five apnea tests were interrupted before the end at 10 min due to rapid desaturation (SatO2 < 90%), with no invalidated apnea test. Mean and standard deviation of blood gas tests assessed before the first apnea test were: pH 7.35(± 0.10), PaO2 252.15 mm Hg (± 114.11), PaCO2 42.78 mm Hg (± 10.84); after the first apnea test: pH 7.11(± 0.08), PaO2 208.39 mm Hg (± 112), PaCO2 82.43 mm Hg (± 16.91); before the second test: pH 7.33 (± 0.09), PaO2 253.56 mm Hg (± 105.36), PaCO2 43.76 mm Hg (± 9.67); following the second apnea test: pH 7.11 (± 0.10), PaO2 200.1 mm Hg (± 116.45), PaCO2 84.98 mm Hg (± 20.21). The pH, PaO2, and PaCO2 values before and after the first and second apnea tests have shown statistically significant differences (P < 0.0001).
Conclusions: The apnea test was safe, blood gas test results are similar to those described in the literature, severe hypoxemias were prevented by a quick reconnection to the mechanical ventilation; and marked hypercapnia and acidemia following the apnea test were found, but no test was invalidated.
Keywords: Brain death; Brain death protocols; Apnea test; Hypercapnia; Acidemia
| Introduction | ▴Top |
After the cause of the coma is identified, the total and irreversible interruption of all brain functions means clinical and legal death [1]. During the development of this work, the diagnosis of brain death (BD) was conducted according to the the Conselho Federal de Medicina (Brazilian Federal Medical Council (CFM)) Decision No. 1,480, August 21, 1997. Once a non-reflexive coma is diagnosed, the protocol establishes that two clinical examinations should be performed, plus two apnea tests (ATs), and ancillary tests [2] with a minimum 6-h time interval for adult patients. The AT is deemed indispensable for diagnosing BD. It should be rigorous enough to prove that there are no respiratory movements evoked by maximal respiratory center stimulation (PaCO2 > 55 mm Hg). Potential complications are related to harmful effects from prolonged exposure to acidemia, hypercapnia, or hypoxemia. Low blood pressure, arrhythmias, and/or hypoperfusion, which may lead to interrupting the test and impairment of organ function are mentioned. This condition may impact the quality and survival of the organs to be donated [3]. In this trial, we aimed to assess the safety of ATs in patients undergoing a BD protocol.
| Materials and Methods | ▴Top |
This was an observational, retrospective cohort study, based on analysis of the continued registration data bank from the intensive care unit (ICU) of the Hospital das Clinicas, State University of Campinas (HC-UNICAMP) and the Transplant Organ Search Organization, in compliance with the Brazilian Federal Medical Council Decision No. 1,480/97, from 2010 to 2017.
In this convenience sample, all consecutively admitted patients eligible for a diagnosis of BD, and with complete clinical records, were included. The inclusion criteria were: age above 13 years, established etiology of coma (Glasgow coma scale = 3), absence of severe endocrine and metabolic disorders possibly causing coma, body temperature above 35 °C, hemodynamic stability with systolic blood pressure (SBP) above 90 mm Hg (even if under vasoactive drugs), and no sedative, hypnotic and/or neuromuscular blocking drugs [2]. Patients with incomplete clinical records and with a cardiorespiratory arrest before the protocol completion were excluded.
From the initial sample of 102 notifications of BD, 87 protocols were evaluated (Fig. 1). The sample profile was characterized by descriptive statistics of the numerical variables with their respective mean and standard deviation values. Intergroup comparisons were made using the Student’s t-test. The trial project was approved by the UNICAMP Institutional Ethics Committee (CAAE #83229417.0.0000.5404). This study was conducted according to the STROBE Statement checklist for cohort studies, the Equator network.
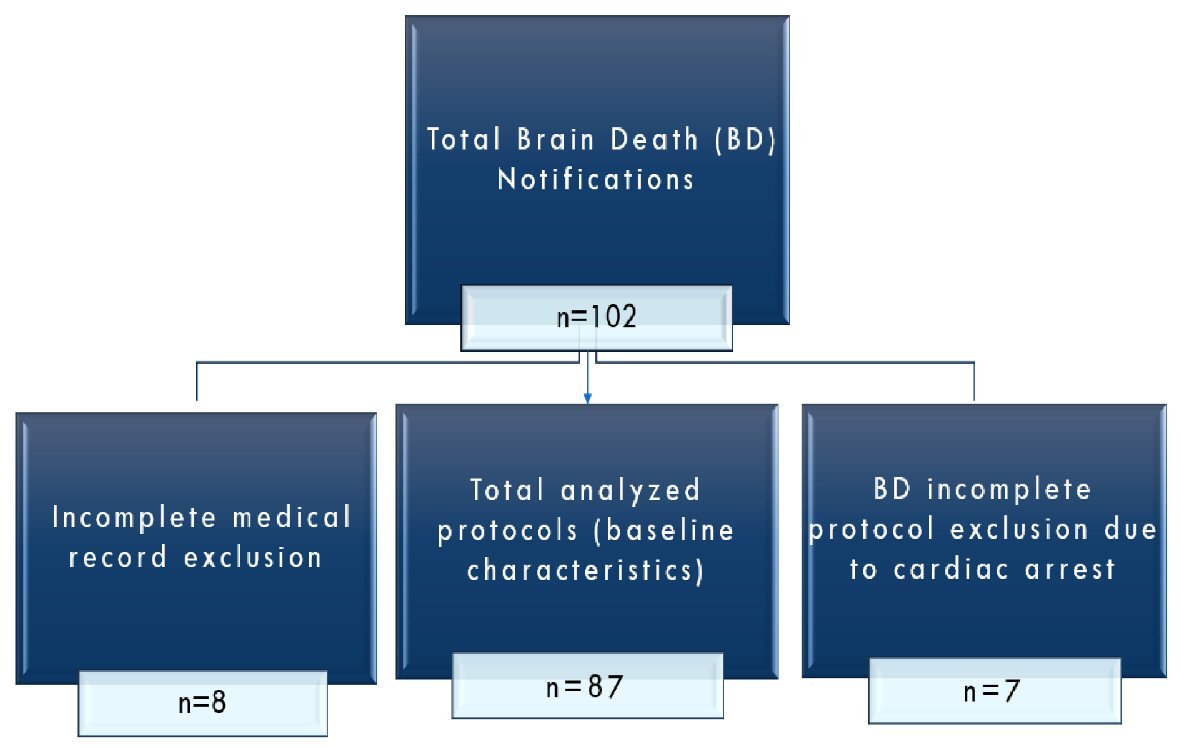 Click for large image | Figure 1. Flowchart of protocols. |
| Results | ▴Top |
The study population characteristics are displayed in Table 1.
 Click to view | Table 1. Population Characteristics |
Eighty-seven protocols were evaluated. Within our population, the main age was 42 (± 16) years old and mostly male individuals 66% (n = 57). The most common coma etiology was traumatic brain injury (n = 38) followed by vascular causes (hemorrhagic stroke, n = 20, brain aneurysm, n = 7 and ischemic stroke, n = 7). Both sodium (Na 154 ± 13 mEq/L) and hemoglobin (Hb) (10 ± 2 mg/dL) were measured previously in the protocol.
The mean AT duration was 11 min. All of the patients were under vasoactive drugs. Only five ATs were interrupted before the end at 10 min due to rapid desaturation (SatO2 < 90%), with no invalidated AT.
The assessed variables were pH, HCO3, PaO2, PaCO2, and SatO2, both before and after the AT. The differences between the first and second ATs are shown in Figures 2 and 3.
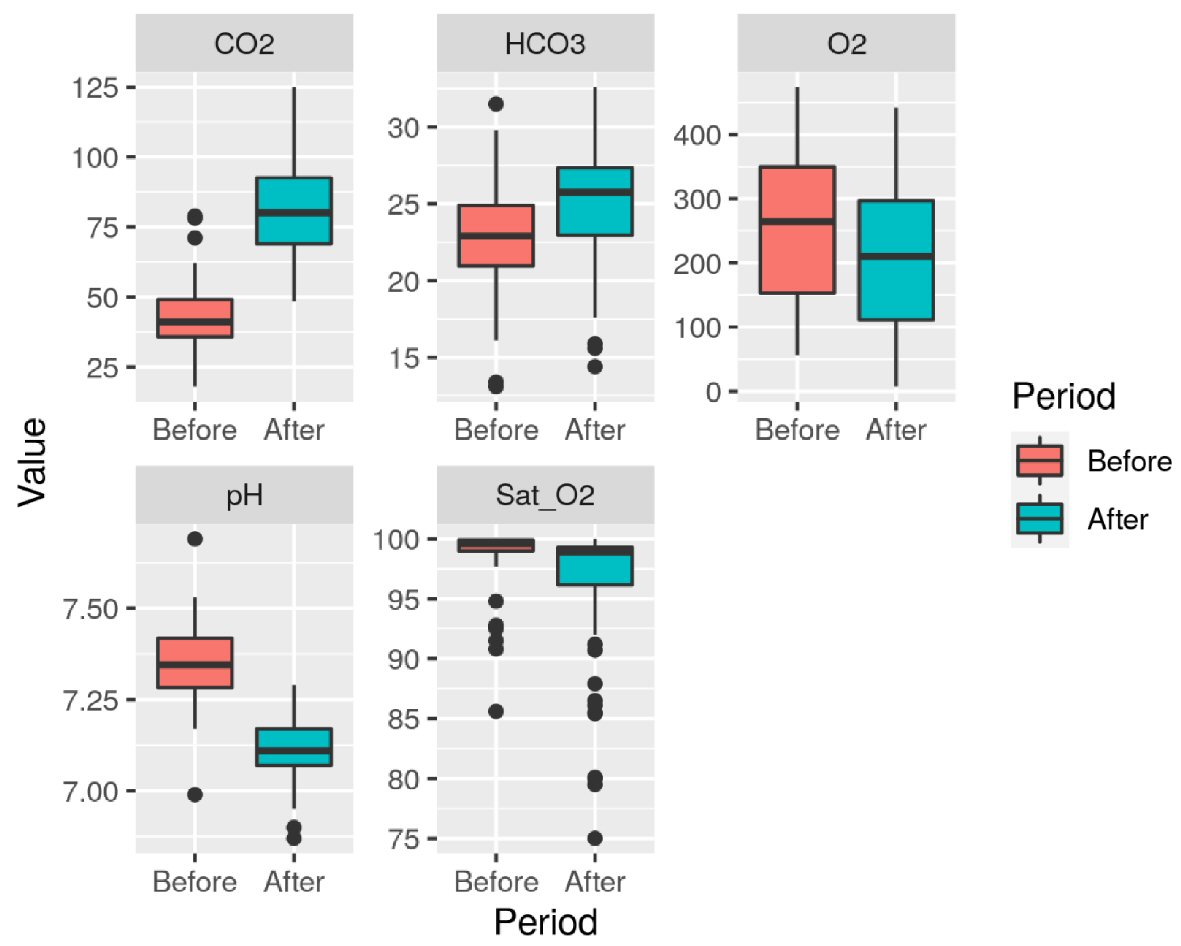 Click for large image | Figure 2. Variables (pH, HCO3, PaCO2, PaO2, and SatO2) distribution in each phase of the first apnea test. Unit: PaO2 (mm Hg), PaCO2 (mmHg), HCO3 (mEq/L), SatO2 (%). |
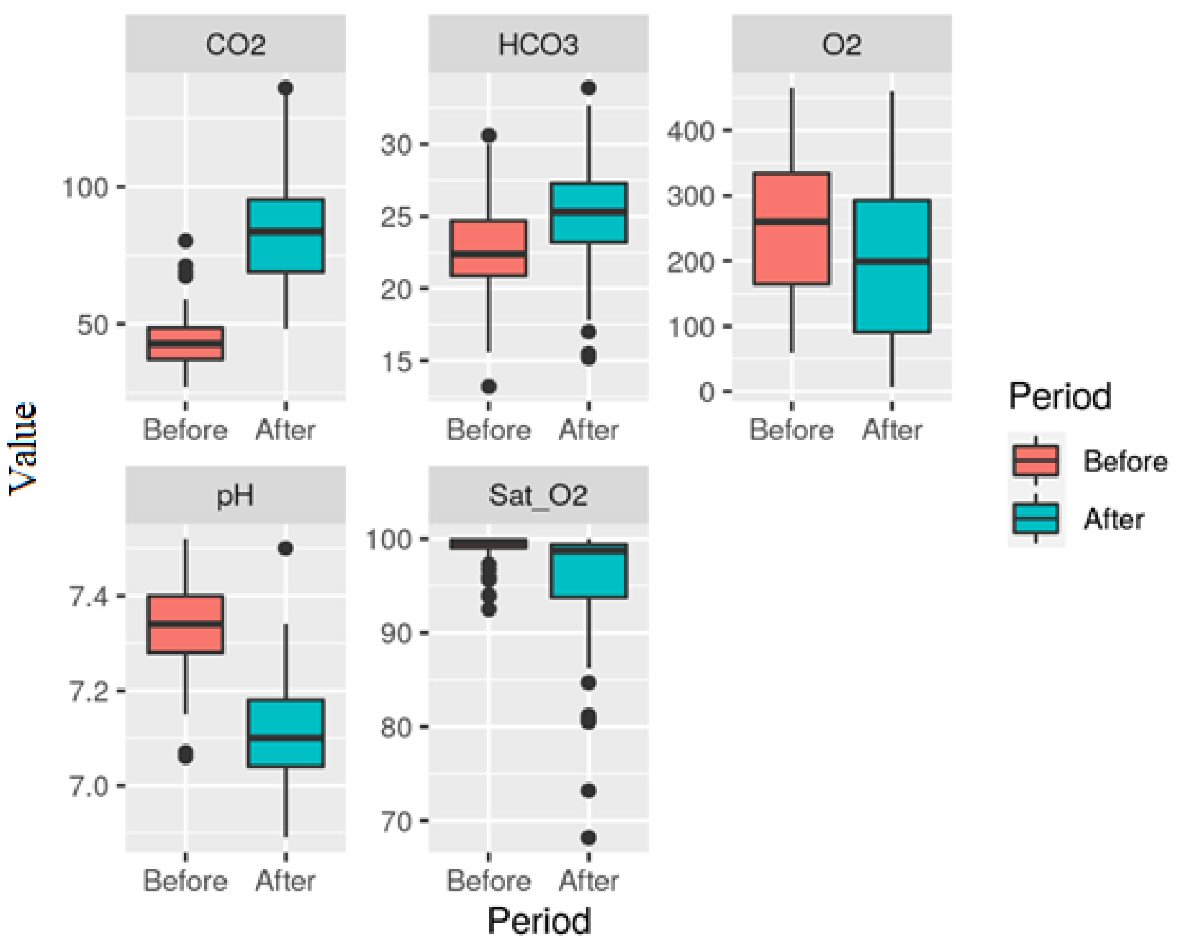 Click for large image | Figure 3. Variables (pH, HCO3, PaCO2, PaO2, and SatO2) distribution in each phase of the second apnea test. Unit: PaO2 (mm Hg), PaCO2 (mmHg), HCO3 (mEq/L), SatO2 (%). |
Mean and standard deviation of blood gas tests assessed before the first AT were: pH 7.35 (± 0.10), PaO2 252.15 mm Hg (± 114.11), PaCO2 42.78 mm Hg (± 10.84), HCO3 22.77 mEq/L (± 3.82), SatO2 98.77% (± 2.44). After the first AT, we found: pH 7.11 (± 0.08), PaO2 208.39 mm Hg (± 112), PaCO2 82.43 mm Hg (± 16.91), HCO3 25.2 mEq/L (± 3.87), SatO2 96.31% (± 5.41). Before the second AT: pH 7.33 (± 0.09), PaO2 253.56 mm Hg (± 105.36), PaCO2 43.76 mm Hg (± 9.67), HCO3 22.66 mEq/L (± 3.5), SatO2 99.09% (± 1.48). Following the second AT: pH 7.11 (± 0.10), PaO2 200.1 mm Hg (± 116.45), PaCO2 84.98 mm Hg (± 20.21), HCO3 25.13 mEq/L (± 3.81), SatO2 95.14% (± 6.86).
Increased PaCO2 values (above 55mm Hg) and low pH (< 7.2) levels were found. The pH, PaO2, and PaCO2 values before and after the first and second ATs have shown statistically significant differences (P < 0.0001).
| Discussion | ▴Top |
During this trial, the diagnosis of BD included two neurological examinations with a minimum 6-h interval, with two ATs following each neurological assessment, and ancillary tests [2]. In December 2017 the Brazilian Federal Medical Council has issued the Decision 2.173; the main changes in this document included reducing to 1 h the minimal time interval between the first and the second clinical assessments and performing one single AT [4, 5]. It is known that coma patients progressing to BD have increased catecholamine and cytokine release, in addition to triggering of the inflammatory cascade. These changes disrupt homeostasis, leading to hemodynamic instability and organ dysfunctions. This physiological deterioration may, in case of donation, impact graft viability [6-9]. Additionally, during the AT, to reach the diagnosis of a definitive respiratory center impairment, it is required to increase the PaCO2. Systemic acidosis, required for maximal respiratory center stimulation, causes detrimental hemodynamic effects, such as myocardial depression, hypotension, and arrhythmias [9-15]. The test methodology, either by apneic oxygen diffusion or positive end-expiratory pressure, reduces hypoxemia and desaturations, however, has no impact on CO2 increase [3, 12].
Regarding PaO2 pre- and post- the first and second ATs, we found a statistically significant difference (P < 0.0001), however, within the boundaries of safety (Figs. 2, 3). During this study, only five ATs were interrupted, two of them in the same patient, before 10 min due to rapid desaturation (SatO2 < 90%). It should be stressed that for safely conducting the AT, some pre-requisites should be in place, such as appropriate medical training [13, 16, 17], and that some authors as Mascia et al [18] advocate performing AT with continuous positive airway pressure therapy (CPAP), based on less physiological repercussion on the donor-to-be and improved short- and long-term transplanted organs survival [19].
As previously reported, the resulting PaCO2 increase and acidemia may cause detrimental systemic effects. Reduced cardiac contractility (especially with pH < 7.20), arrhythmias (also due to increased serum potassium), reduced endogenous and exogenous cardiovascular response to catecholamines, increased pulmonary vasoconstriction (increasing right ventricular afterload), in addition to the cell function itself, are mentioned. Studies suggest that a pH < 7.20 causes deep inhibition of intracellular phosphokinase production and decreased cellular energy production [3, 20].
During the AT, PaCO2 displays a biphasic behavior, with a marked increase during the first minutes, up to 5 mm Hg/min and, later, ranging from 2 - 3 mm Hg/min [3]. Based on this, one should avoid starting an AT with PaCO2 values below 35 mm Hg, as it would imply longer apnea time, and therefore, increased exposure to higher CO2 levels and acidemia caused by the respiratory acidosis and the mentioned detrimental effects, added to a risk of hypoxemia [21]. CO2 level may be better assessed by using end-tidal capnometry during the pre-oxygenation phase, and/or using transcutaneous carbon dioxide partial pressure monitoring, which would provide a better CO2 increase assessment during apnea [21, 22].
We found increased PaCO2 values (above 55 mm Hg) and low pH (< 7.2) levels, however with no AT interruption or invalidation due to hemodynamic instability caused by acidemia. It should be noted that 100% of the patients were under vasoactive drugs (Table 1).
Based on this cohort, we conclude that the AT was safe. The blood gas parameters observed are similar to those mentioned in the literature. We stress that despite the hypercapnia and acidemia observed by the end of the AT, no test was invalidated due to hypotension not responding to vasoactive drugs (SBP < 90 mm Hg), arrhythmias or hypoxemia.
This study highlights the safety of the AT. The inclusion of a single AT [4, 5] prevents unnecessary exposure to acidemia, hypercarbia, risk of hypoxemia, as well as the risk of cardiovascular and barotrauma complications [23].
This study has limitations. The observational and retrospective design, the lack of objective tests showing myocardial depression, the lack of measuring vasoactive drugs dose changes during the AT, and the lack of description of AT methods, should be mentioned.
Acknowledgments
We would like to acknowledge the contribution of the Intensive Care Unit and Transplant Organ Search Organization multidisciplinary teams, especially Claudineia Logatto, Rafaela Batista dos Santos Pedrosa, Klenio de Oliveira Bonfim, Luciana Aparecida dos Santos, and Maria Valeria de Omena Athayde.
Financial Disclosure
This study had no funding source.
Conflict of Interest
No conflict of interest to be reported.
Informed Consent
Ethical clearance for such a retrospective analysis was provided by the institutional ethical committee but informed consent was not asked. The data were collected secondarily through the database and medical records and not directly with individuals or family members, in this way, respecting confidentiality, without causing harm to the patient or institution.
Author Contributions
TS conceived and designed the study, analyzed the data, and with NN’s contribution wrote the first and revised version of the manuscript. GF contributed to the study data analysis and interpretation. RM and AB contributed to reviewing critically data analysis and manuscript. VF, HZ, APG, and LAS contributed with manuscript writing and revision. ALF contributed substantially to important intellectual content with study design, manuscript writing, revision, and final approval of the version to be published. All authors read and approved the final manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- A definition of irreversible coma. Report of the Ad Hoc Committee of the Harvard Medical School to examine the definition of brain death. JAMA. 1968;205(6):337-340.
doi - Conselho Federal de Medicina, Resolucao CFM no 1.480/1997. https://sistemas.cfm.org.br/normas/visualizar/resolucoes/BR/1997/1480.
- Solek-Pastuszka J, Biernawska J, Iwanczuk W, Kojder K, Chelstowski K, Bohatyrewicz R, Sawicki M. Comparison of two apnea test methods, oxygen insufflation and continuous positive airway pressure during diagnosis of brain death: final report. Neurocrit Care. 2019;30(2):348-354.
doi pubmed - Conselho Federal de Medicina, Resolucao CFM no 2.173/2017. https://sistemas.cfm.org.br/normas/visualizar/resolucoes/BR/2017/2173.
- Westphal GA, Veiga VC, Franke CA. Diagnosis of brain death in Brazil. Rev Bras Ter Intensiva. 2019;31(3):403-409.
doi pubmed - Opdam HI. Hormonal Therapy in Organ Donors. Crit Care Clin. 2019;35(2):389-405.
doi pubmed - Jennett B, Gleave J, Wilson P. Brain death in three neurosurgical units. Br Med J (Clin Res Ed). 1981;282(6263):533-539.
doi pubmed - Wijdicks EF. Management of the comatose patient. Handb Clin Neurol. 2017;140:117-129.
doi pubmed - Scott JB, Gentile MA, Bennett SN, Couture M, MacIntyre NR. Apnea testing during brain death assessment: a review of clinical practice and published literature. Respir Care. 2013;58(3):532-538.
doi pubmed - Lang CJ, Heckmann JG. Apnea testing for the diagnosis of brain death. Acta Neurol Scand. 2005;112(6):358-369.
doi pubmed - Shutter L. Pathophysiology of brain death: what does the brain do and what is lost in brain death? J Crit Care. 2014;29(4):683-686.
doi pubmed - Datar S, Fugate J, Rabinstein A, Couillard P, Wijdicks EF. Completing the apnea test: decline in complications. Neurocrit Care. 2014;21(3):392-396.
doi pubmed - Salih F, Hoffmann O, Brandt SA, Masuhr F, Schreiber S, Weissinger F, Rocco A, et al. Safety of apnea testing for the diagnosis of brain death: a comprehensive study on neuromonitoring data and blood gas analysis. Eur J Neurol. 2019;26(6):887-892.
doi pubmed - Russell JA. Author response: Brain death, the determination of brain death, and member guidance for brain death accommodation requests: AAN position statement. Neurology. 2019;93(21):948.
doi pubmed - Daneshmand A, Rabinstein AA, Wijdicks EFM. The apnea test in brain death determination using oxygen diffusion method remains safe. Neurology. 2019;92(8):386-387.
doi pubmed - Bernat JL, Brust JCM. Strategies to improve uniformity in brain death determination. Neurology. 2019;92(9):401-402.
- Braksick SA, Robinson CP, Gronseth GS, Hocker S, Wijdicks EFM, Rabinstein AA. Variability in reported physician practices for brain death determination. Neurology. 2019;92(9):e888-e894.
- Mascia L, Pasero D, Slutsky AS, Arguis MJ, Berardino M, Grasso S, Munari M, et al. Effect of a lung protective strategy for organ donors on eligibility and availability of lungs for transplantation: a randomized controlled trial. JAMA. 2010;304(23):2620-2627.
doi pubmed - Michel SG, Madariaga MLL, LaMuraglia GM, 2nd, Villani V, Sekijima M, Farkash EA, Colvin RB, et al. The effects of brain death and ischemia on tolerance induction are organ-specific. Am J Transplant. 2018;18(5):1262-1269.
doi pubmed - Powner DJ, Kellum JA. Maintaining acid-base balance in organ donors. Prog Transplant. 2000;10(2):98-103; quiz 104-105.
doi pubmed - Busl KM, Lewis A, Varelas PN. Apnea Testing for the Determination of Brain Death: A Systematic Scoping Review. Neurocrit Care. 2020.
doi - Vivien B, Marmion F, Roche S, Devilliers C, Langeron O, Coriat P, Riou B. An evaluation of transcutaneous carbon dioxide partial pressure monitoring during apnea testing in brain-dead patients. Anesthesiology. 2006;104(4):701-707.
doi pubmed - Goudreau JL, Wijdicks EF, Emery SF. Complications during apnea testing in the determination of brain death: predisposing factors. Neurology. 2000;55(7):1045-1048.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.
