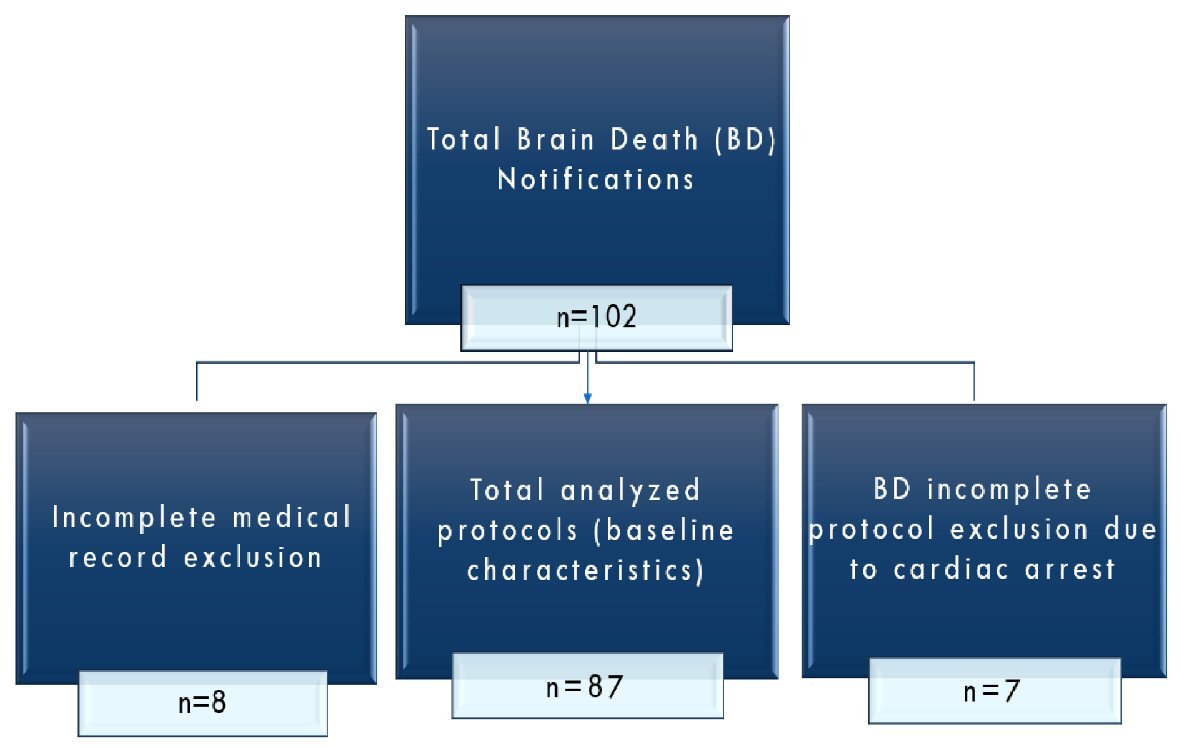
Figure 1. Flowchart of protocols.
| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website https://www.neurores.org |
Original Article
Volume 11, Number 1-2, April 2021, pages 14-19
Apnea Test Safety in Brain Death: A Single-Center Retrospective Cohort Analysis
Figures

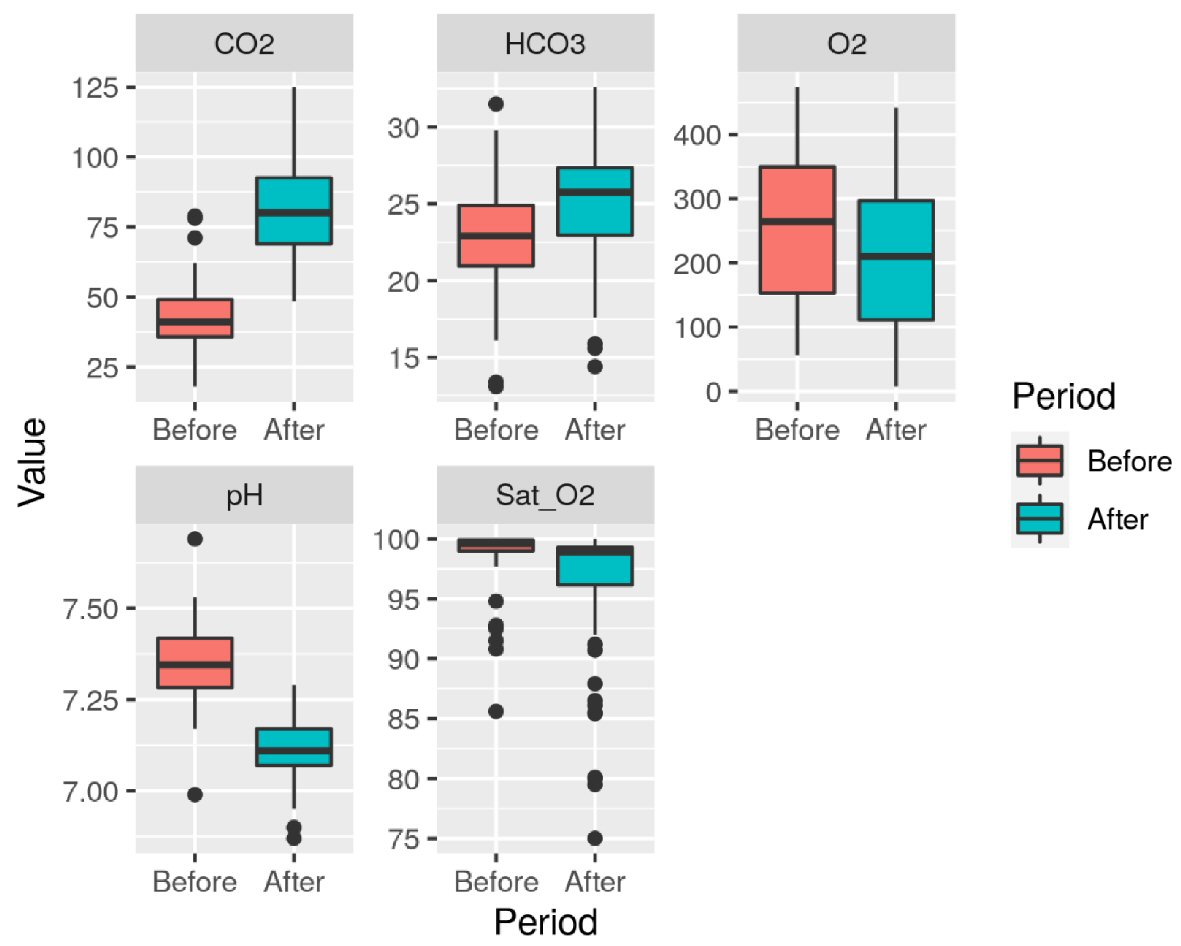
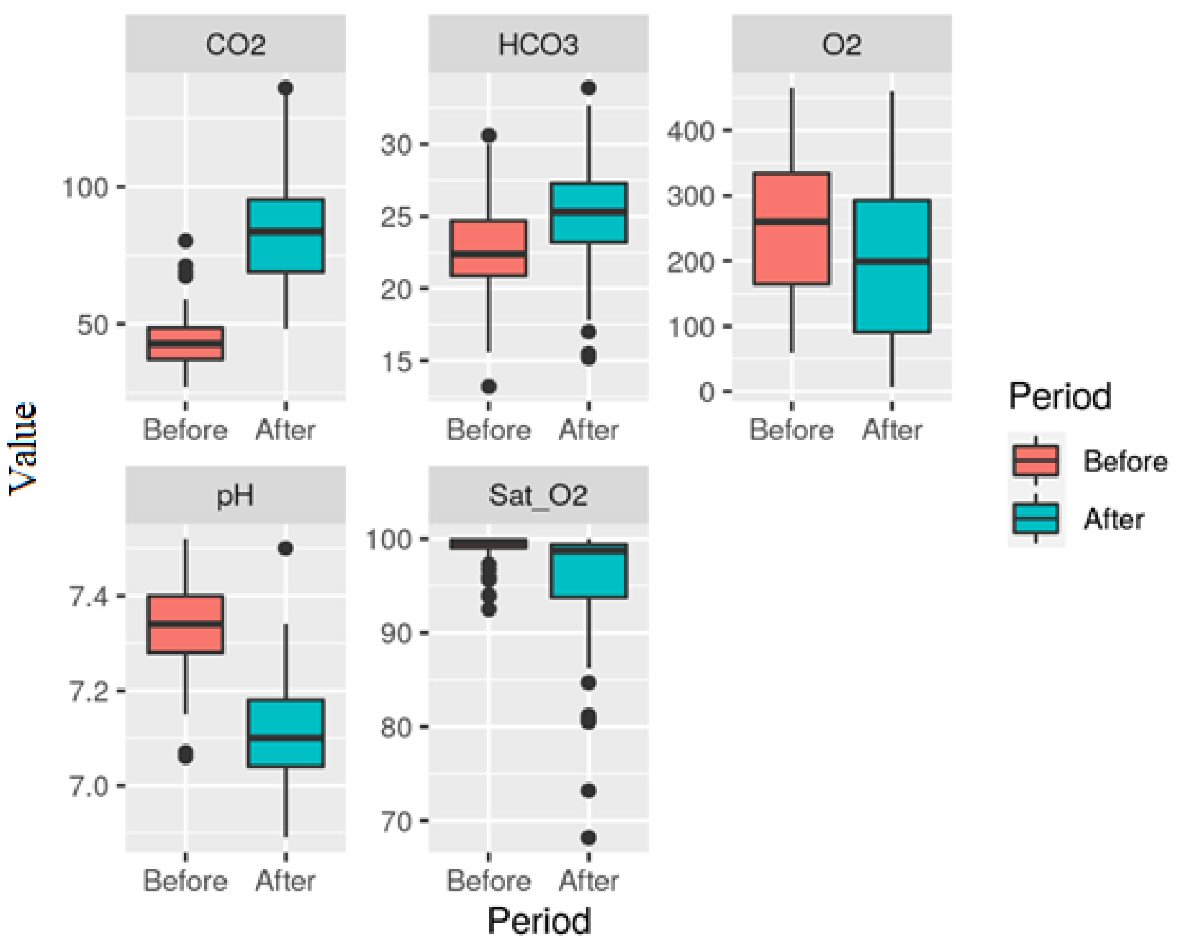
Table
| Characteristics | N = 87 |
|---|---|
| AT: apnea test; SD: standard deviation; ICU: intensive care unit; SOFA: sequential organ failure assessment; APACHE: acute physiology and chronic health evaluation; SAPS: simplified acute physiology score. | |
| Age, years | |
| Mean | 42 |
| SD | 16 |
| Sex, n (%) | |
| Male | 57 (66%) |
| Female | 30 (34%) |
| Coma etiology, n | |
| Traumatic brain injury | 38 |
| Hemorrhagic stroke | 20 |
| Brain aneurysm | 10 |
| Brain tumor | 9 |
| Ischemic stroke | 7 |
| Anoxic encephalopathy | 2 |
| Sepsis | 1 |
| Sodium (before AT), mEq/L | |
| Mean | 154 |
| SD | 13 |
| Hemoglobin, mg/dL | |
| Mean | 10 |
| SD | 2 |
| Duration of 1st AT, min | |
| Mean | 11 |
| SD | 2 |
| Duration of 2nd AT, min | |
| Mean | 11 |
| SD | 2 |
| BD protocol duration, h | |
| Mean | 15 |
| SD | 8 |
| Use of vasoactive drugs, n (%) | |
| Yes | 87 (100%) |
| ICU admission SOFA | |
| Mean | 8 |
| SD | 4 |
| ICU admission APACHE II | |
| Mean | 20 |
| SD | 7 |
| ICU admission SAPS 3 | |
| Mean | 54 |
| SD | 15 |