| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website http://www.neurores.org |
Original Article
Volume 2, Number 1, February 2012, pages 1-9
Temozolomide and/or Erlotinib in the Treatment of Lung Cancer Patients With Progressive Central Nervous System Metastases
Rimas V Lukasa, c, Martin Kelly Nicholasa, b, Victoria Villaflorb, Philip C. Hoffmanb, Ravi Salgiab
aDepartment of Neurology, University of Chicago, USA
bDepartment of Medicine-Section of Hematology-Oncology, University of Chicago, USA
cCorresponding author: Rimas V Lukas, Department of Neurology, University of Chicago, 5841 South Maryland Avenue, MC 2030, Chicago, Illinois 60637, USA
Manuscript accepted for publication February 21, 2012
Short title: Temozolomide and/or Erlotinib in Lung Cancer
doi: https://doi.org/10.4021/jnr85w
| Abstract | ▴Top |
Background: Patients with lung cancer who develop brain metastases have a poor prognosis. Those patients with progressive brain metastases tend to have a dismal prognosis. Currently, there is no standard of care for the treatment of these patients.
Methods: In this manuscript, we present a retrospective evaluation of 10 patients treated at our institution with a combination of temozolomide and/or erlotinib after disease progression in the central nervous system following radiation therapy.
Results: Median overall survival was 28 weeks. Median time to progression in the central nervous system was 14 weeks. Median time to progression systemically was 7.5 weeks. Some patients demonstrated prolonged stability of disease.
Conclusions: A palliative regimen of temozolomide and/or erlotinib could be considered in progressive central nervous system metastases from lung cancer.
Keywords: Lung cancer; Brain metastases; Temozolomide; Erlotinib; Epidermal growth factor receptor
| Introduction | ▴Top |
The most common central nervous system (CNS) malignancy is metastatic disease. Lung cancer accounts for approximately half of all brain metastases [1, 2]. The presentation of brain metastases is often metachronous with a latency of 6 to 9 months after initial cancer diagnosis [3]. Survival in these patients remains poor. Median overall survival (OS) in lung cancer patients once they develop CNS metastases is approximately 6 months [3-5]. The prognosis is poorer in small-cell lung cancer (SCLC) compared to non-small cell lung cancer (NSCLC) [5]. The majority of patients with brain metastases have multiple lesions [6]. In turn, they receive radiation therapy as their initial treatment. At the time of progression in the CNS after initial therapy, the prognosis is deemed to worsen although data is limited [7-10]. Survival in brain metastases patients is strongly affected by factors such as age, performance status, control of primary tumor, and presence of extracranial metastases [5, 11]. Chemotherapy trials for progressive brain metastases in general report median OS ranging from 3.5 to 6.6 months. Currently there is no standard of care for progressive brain metastases in general, or for progressive brain metastases from lung cancer specifically [12].
The frequent presence of concomitant active systemic disease and progressive multiple CNS metastases is a scenario which could potentially benefit from administration of systemic therapy to address all sites of disease. This is a challenging issue as most systemic therapies which are effective in lung cancer do not cross the blood brain barrier (BBB) at effective concentrations. The BBB limits the transfer of larger or more hydrophilic molecules into the CNS. It also precludes agents which are substrates for the multi drug resistance gene products from reaching adequate concentrations in the CNS [13]. This study addresses these concerns by combining temozolomide (Merck, Whitehouse Station, USA) and erlotinib (Genentech, San Francisco, USA; OSI Pharmaceuticals, Melville, USA; Roche, Basel, Switzerland) in an attempt to address treatment of CNS metastatic disease. Both agents cross the BBB to some degree.
Erlotinib is a small molecule epidermal growth factor receptor (EGFR) specific tyrosine kinase inhibitor (TKI) used in the treatment of systemic NSCLC [9]. CSF concentrations in relation to serum concentrations of erlotinib and its active metabolite OSI-420 range from 1 to 7% and 3 to 9% respectively, depending on the dosing schedule [14, 15]. The tumor tissue to plasma ratio of erlotinib and OSI-420 at trough steady state levels in patients with high-grade gliomas is 0.38 and 0.48 respectively [16]. This agent may preferentially accumulate in highly vascular tumors such as glioblastoma. In turn, the concentrations of this agent in these tumors may not represent concentrations achieved in lung cancer brain metastases. However, uptake of erlotinib in NSCLC brain metastases has been demonstrated via positron emission tomography [17].
EGFR TKI has been studied for potential benefit in patients with NSCLC involving the CNS. Patients of Asian ancestry are more likely to respond to EGFR TKI. There are case reports of patients from this population who had responses to treatment and even retreatment of CNS parenchymal metastases and leptomeningeal metastases (LM). Some of these cases have resulted in meaningful response times up to 8 months [18-22].
Two retrospective case series (28 patients total) from Japan evaluating the response of NSCLC brain metastases to the EGFR TKI gefitinib demonstrated evidence for response in the CNS [23, 24]. Another recent retrospective series (n = 93) of NSCLC brain metastases patients conducted in the U.S. looked at the role of EGFR mutation on outcome. A small number of patients (n = 6) were treated with an EGFR TKI as front-line therapy for their brain metastases. Sustained complete response (CR) was observed in 2 of the patients with EGFR-mutations. The exact mutations in these patients were not described. No response was seen in the single wild-type EGFR patient treated with upfront EGFR TKI for brain metastases. In the larger patient population with brain metastases who received EGFR TKI after their initial brain-directed treatment both OS and time to progression in the CNS (TTP-CNS) were improved compared to those who did not receive EGFR TKI. Selection bias favored mutant EGFR patients to receive EGFR TKI. Of note, there were no African American (AA) patients in this study [25]. These studies were complicated by including patients who had received WBRT, stereotactic radiosurgery (SRS), WBRT and SRS, as well as those who had not received radiation therapy to the brain [23-25]. Two prospective trials conducted in Asia using EGFR TKI in newly diagnosed brain metastases have recently been published. One phase II trial treated patients with WBRT (40 Gy in 20 fractions) with concomitant gefitinib (250 mg daily) during WBRT and continuously thereafter, 19% CR and 62% partial response (PR) were noted. Median progression free survival (PFS) was 10.0 months and median OS was 13.0 months. Females and never smokers were more likely to respond [26]. Of note, these patients are the most likely to have EGFR mutations associated with responses. Another trial including only Asian never smokers with adenocarcinoma of the lung, synchronous brain metastases, and no prior radiation therapy treated with either erlotinib (150 mg daily) or gefitinib (250 mg daily) noted no CR but did have 69.6% PR. PFS was 7.1 months and OS 18.8 months [27]. This trial raises the possibility of delaying WBRT with the use of EGFR specific TKIs in selected patients. Finally a prospective study performed in Italy looked at patients with NSCLC brain metastases, almost half of whom had received WBRT, treated with gefitinib. The radiographic response rate was much less robust (0% CR, 10% PR) as was the PFS (3 months). Most of the patients were male (71%) and the smoking history was not reported. The difference between the results of this trial and those performed in Asia may be due to patient selection and the likelihood of possessing an EGFR mutation. The trial conducted in Italy enrolled both patients with newly diagnosed brain metastases as well as the poorer prognosis group of those with brain metastases which progressed after WBRT. Additionally, Asian female non-smokers in the first two trials have a higher likelihood to respond to EGFR TKI than the patient population in the Italian study [28].
Temozolomide is an oral methylating imidotetrazione with good CNS penetration used in the treatment of high-grade gliomas [29]. Concentrations of temozolomide in the cerebrospinal fluid (CSF), a surrogate for CNS concentrations, are approximately 20% of serum concentrations [30]. Cases reporting the use of temozolomide in combination with other chemotherapies have noted clinical and radiographic improvement in CNS disease from both SCLC and NSCLC [31]. In a phase II study of recurrent brain metastases (54% NSCLC, 5% SCLC) using temozolomide on a 5 out of 28 day schedule the NSCLC patients demonstrated PR 9.1% and stable disease (SD) 36.4%. Median TTP (either CNS or systemic) for the NSCLC patients was 3.18 months [10]. A number of trials using temozolomide for newly diagnosed brain metastases have been published. One trial exclusively looking at NSCLC patients with brain metastases treated with WBRT vs WBRT with concomitant temozolomide followed by two cycles of adjuvant temozolomide demonstrated apparent safety and tolerability of the combined regimen. Although there was no improvement in OS with combination therapy, there was improvement in 90 day progression free survival (PFS) in the CNS (54% vs 72%, P = 0.03) and incidence of neurologic death (69% vs 41%, P = 0.03) [32]. A similar trial of patients with various solid tumors (60% NSCLC, 17% SCLC) using WBRT with concomitant temozolomide followed by six adjuvant cycles of temozolomide demonstrated improved radiographic responses (CR 38% vs 33%, PR 58% vs 33%) in the chemoradiation arm. The improvement in OS in the chemoradiation arm was not statistically significant [33].
| Materials and Methods | ▴Top |
This is a retrospective study in which we reviewed the Thoracic Oncology database at the University of Chicago for patients with lung cancer and brain metastases treated with erlotinib, temozolomide, or a combination of the two. Ten patients with progressive CNS metastases after RT who initiated treatment with temozolomide and/or erlotinib between 1/2005 and 1/2010 met these criteria. Charts and images were reviewed for demographic and clinical data as well as extent and duration of response systemically and in the CNS. Written consent via an IRB approved protocol was obtained from all living patients.
OS was measured from the first date that MRI CNS imaging revealed CNS progression which led to the initiation of temozolomide and/or erlotinib. If exact date of death was not documented in our records, the Social Security Death Index database was searched. If the exact death date could not be obtained in this way (n = 1, patient #6), the most recent date in our records was used as the date of death. For patients still alive, OS was censored on 7/26/2010. TTP-CNS was calculated from the date of the MRI CNS imaging revealing progressive CNS metastases leading to the initiation of temozolomide and/or erlotinib until the CNS imaging documented PD or until death or loss to follow-up. TTP systemically (TTP-S) was calculated from the date of the same MRI CNS imaging used to calculate TTP-CNS until systemic imaging revealed PD or until death or loss to follow-up. If patients were lost to follow-up, the most current date in our medical records was used to calculate TTP-CNS and TTP-S. CNS disease was monitored primarily with MRI. In a limited number of patients, MRI imaging was unable to be performed and CT was used in those settings. The data from one patient (patient #10) was not used in calculating duration of CNS response due to inability to tolerate erlotinib. MRI 2 weeks after initiating therapy was stable. In evaluating systemic progression CT and/or PET imaging was used. Imaging was not consistently obtained if the patient’s condition deteriorated significantly prior to death. In these scenarios (n = 3) the date of the most recent systemic staging was used to calculate systemic TTP. If these patients had a short TTP systemically our calculations would be overestimating the TTP-systemic.
| Results | ▴Top |
We reviewed the records from our Thoracic Oncology Clinic for lung cancer patients with CNS metastases treated with temozolomide and/or erlotinib. Ten patients (5 male, 5 female) were treated from 2005 to 2010 (Table 1). Half of the patients were female. The mean age was 54. All patients were smokers. None was of Asian ancestry. Three were AA and seven were Caucasian (C). Eight patients had NSCLC and two had SCLC. One patient had leptomeningeal disease (positive CSF cytology) in addition to parenchymal brain metastases. For another patient there was strong clinical suspicion for LM in addition to parenchymal brain metastases, but negative CSF and imaging. For a third patient there was clinical suspicion for LM accompanied by abnormal CSF findings but negative CSF cytology and MRI without radiographic evidence of LM. Two of the patients received additional chemotherapy during their regimen of temozolomide and/or erlotinib. One patient received temozolomide with paclitaxel for his systemic disease. Another patient received irinotecan (Pfizer, New York, USA) with temozolomide (Table 1). Prior malignancies in these patients included local basal cell skin cancer (n = 1), ductal carcinoma in situ (DCIS) of the breast (n = 1), and locally advanced high-grade transitional cell cancer of the bladder (n = 1). All of these additional malignancies were diagnosed prior to the lung cancer. None of these malignancies was deemed to be active during the course of treatment with erlotinib and/or temozolomide. Two patients were on enzyme-inducing anti-epileptic drugs (EIAED) during the course of treatment. EIAED affect the metabolism of erlotinib, leading to decreased concentrations.
 Click to view | Table 1. Patients’ Data |
Median TTP in the CNS was 14 weeks (n = 9). Median TTP systemically was 7.5 weeks (n = 10). Median OS was 28 weeks (n = 10) (see supplemental file at www.neurores.org). No patients developed CR or PR in the CNS. All patients demonstrated SD in the CNS on their first follow up imaging study after initiating the chemotherapy regimen. Some lesions decreased in size or became more necrotic radiographically but did not meet criteria for PR (Fig. 1).
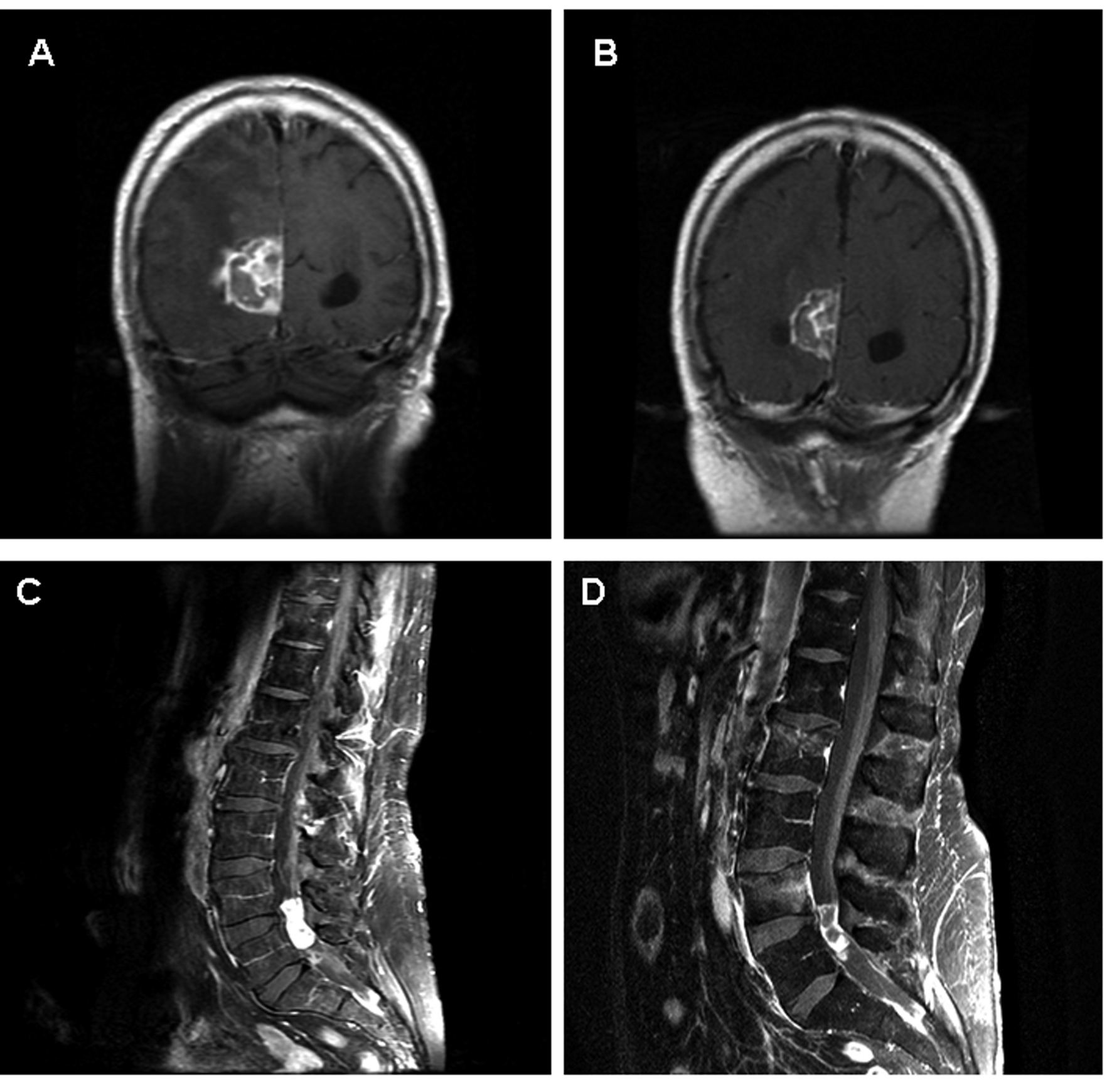 Click for large image | Figure 1. Images A and B represent coronal T1 post contrast MRIs of the brain from patient 2. Image A is prior to initiation of temozolomide/erlotinib. Image B is 8 weeks later demonstrating a substantial decrease in the size of the lesion, but not meeting PR per RECIST criteria. Images C and D represent sagital T1 post contrast MRIs of the lumbar spine from patient 8. Image C is at the time of diagnosis of CSF involvement of NSCLC. Multiple areas of bulky disease were noted throughout the CSF space. Image D demonstrates SD after four months on erlotinib. The area of decreased signal within the tumor may represent necrosis. |
One patient (patient 7) demonstrated a prolonged sustained response. TTP CNS was 53 weeks. The patient, a female AA smoker, continued to be progression free systemically at 84 weeks. The patient had NSCLC and developed parenchymal brain metastases 4 years after her initial diagnosis of lung cancer, without evidence of systemic recurrence. She had 3 lesions and underwent resection of the largest which had significant associated mass effect. This was followed by WBRT to 30 Gy. When she had radiographic progression at the prior sites of intracranial disease as well as extracranial subcutaneous extension of tumor approximately 8 months after WBRT, she was started on a combination of erlotinib (150 mg daily) and temozolomide (150 mg/m2 for 5 out of 28 days) (Fig. 2). She experienced a mild radiographic improvement, insufficient to be defined as a PR. She also had resolution of her subcutaneous nodules over the course of one month. The patient maintained stable disease for approximately 12 months following initiation of this regimen.
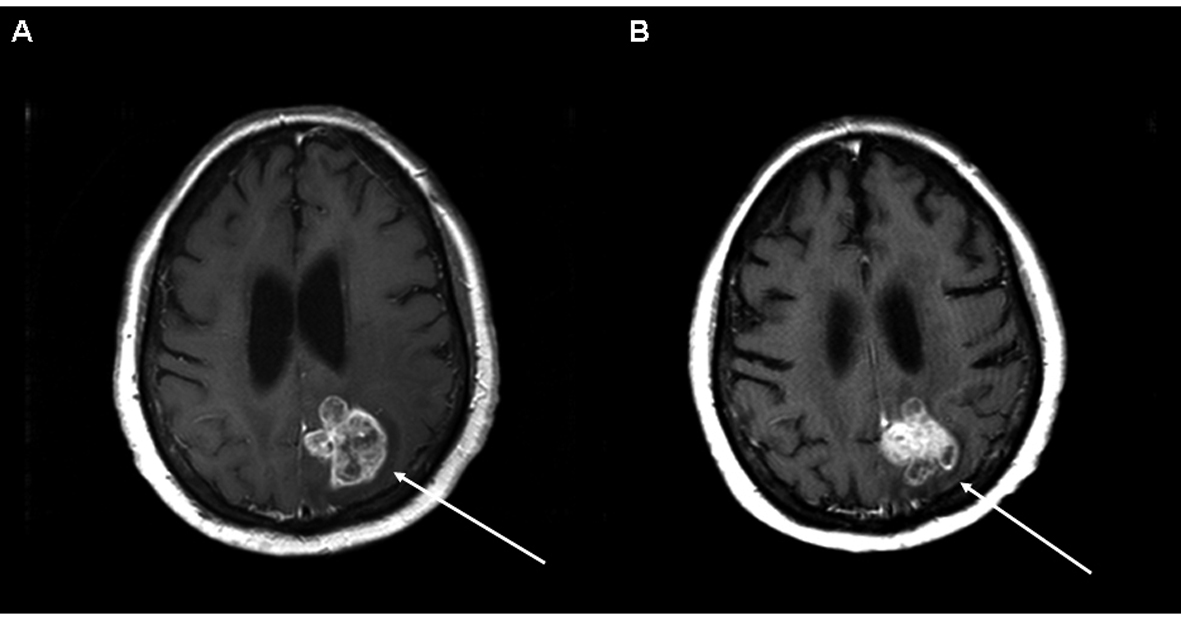 Click for large image | Figure 2. Axial T1 post contrast MRI of the brain from patient 7 demonstrating a large left parietal region brain metastasis. Image A is at the time of initiation of temozolomide/erlotinib. Image B demonstrates SD six months later. |
| Discussion | ▴Top |
CNS metastases from lung cancer are common and are associated with significant morbidity and mortality. Current standard of care for initial therapy for multiple brain metastases regardless of histology involves WBRT. At this time there is no standard of care for LM [12]. There is developing evidence for improved response rates and OS in NSCLC with the addition of systemic chemotherapy to WBRT for newly diagnosed brain metastases [25, 34]. New treatment regimens for brain metastases are needed to prolong survival, time to progression, and quality of life. These regimens will need to be evaluated based on a histology specific basis. Currently treatment should be patient specific and may include palliative chemotherapy [12].
The potential beneficial role of erlotinib in recurrent CNS metastases from lung cancer is limited to NSCLC. Our study included two patients who had SCLC. Reports of parenchymal brain metastases patients treated with erlotinib, particularly those with EGFR mutations, have noted radiographic responses, including sustained CR [18, 19, 25]. Additional cases of LM due to NSCLC have noted cytologic clearing in the CSF and radiographic stability or improvement when erlotinib was used as part of a combination regimen [21, 22]. EGFR TKI has been evaluated prospectively in newly diagnosed brain metastases in combination with WBRT. Both gefitinib and erlotinib have been evaluated and have appeared to be tolerable with suggestion of possible benefit [26, 35].
Our study demonstrates that a combination regimen of erlotinib and temozolomide is a reasonable therapeutic strategy after progression of CNS metastases following WBRT. Our patient population differs from those recently reported in the trials by Ma and Kim [25, 26]. We had a higher proportion of African American patients, who are less likely to respond as robustly to EGFR TKI as the Asian patients in the above noted studies. Our study included only smokers, again a subpopulation less likely to respond to EGFR TKI. Nonetheless, the majority of our patients demonstrated stable disease, and select patients this was sustained for a number of months before acquired resistance developed. The greatest limitation of our study is its retrospective nature. No standardized treatment regimens were used. The majority of patients (60%) received both agents with erlotinib administered at 150 mg daily (in one patient being held on days of temozolomide doses) and temozolomide (150 - 200 mg/m2) for 5 of 28 days. Interestingly, one of the longest responders was also on an EIAED throughout much of treatment, further complicating interpretation of the potential role erlotinib played. The use of EIAEDs can lead to subtherapeutic concentrations of erlotinib. EIAEDs are also, however, associated with an increased conversion of erlotinib to its active metabolite, OSI-420 [36]. All patients previously received WBRT. Another limitation of the retrospective nature of the study was a lack of a standardized imaging protocol. However, the majority were evaluated clinically at regular intervals by a neuro-oncologist (MKN, RVL). All patients were followed with serial contrast-enhanced MRIs of the brain and spine (in the cases of LM). These were typically performed every two months as well as at any times of clinical concern for CNS progression.
Our study of erlotinib and/or temozolomide for lung cancer brain metastases which progressed after RT demonstrated median OS of 28 weeks, median TTP-CNS of 14 weeks, and median TTP-systemic of 7.5 weeks. The TTP-systemic is influenced in part by the 3 patients who did not undergo systemic restaging and skewed the results towards a shorter time interval. Radiographically, all patients demonstrated SD in the CNS. There were no patients with CNS PR or CR. One patient (patient 10) discontinued treatment after one combined cycle of temozolomide/erlotinib, due to perceived side effects of the erlotinib. It is likely that the patients neurologic symptomatology (gait difficulty leading to falls) was more likely due to CNS tumor burden. No patients discontinued therapy due to any other toxicities. Erlotinib and/or temozolomide appear tolerable in the treatment of progressive brain metastases.
The patient population in our study consisted of 70% C and 30% AA patients. There were no patients of Asian ancestry. Half of the patients were female. It has long been recognized that AA patients have a poorer response to lung cancer treatment compared to C. It has also been shown that subgroups of patients with NSCLC are likelier to respond to the EGFR TKI erlotinib [9]. Our two longest responders were AA female smokers. Somatic mutations in the region of the gene encoding the intracellular domain of EGFR confer sensitivity to both erlotinib and gefitinib. These mutations are more commonly found in Asian females with adenocarcinomas [37]. Mutations, such as the double L858R and E884K mutations, have been associated with response in the CNS of a Japanes female with NSCLC treated with one EGFR TKI (gefitinib) after progression on a different prior EGFR TKI (erlotinib) [20]. Further work has been done examining the role of ethnic differences in frequency and type of mutations associated with lung cancer. EGFR somatic mutations have been found at a much higher incidence in East Asian (EA) patients (32%) compared to C and AA (3%). Other tyrosine kinases such as MET have also been found to have higher incidences of germ-line mutations in EA compared to C with an absence of these mutations in AA [38]. As our understanding of the role of both somatic and germline mutations and knowledge of their incidences in various ethnic populations improves we will move closer to patient-specific approaches to therapy in lung cancer.
O6-methylguanine-DNA-methyltransferase (MGMT) is a DNA repair enzyme which removes methyl groups from the O6 position of guanine. Methylation of the MGMT promoters leads to decreased expression of MGMT and in turn greater mutagenicity. MGMT methylation is also associated with a more pronounced response to alkylating agents such as temozolomide in primary brain tumors [29]. A study of MGMT methylation promoter status conducted in Taiwan evaluating brain metastases from lung cancer has demonstrated enhanced MGMT expression in the brain metastases in comparison to the primary tumor. This raises concern for potential lack of response in the CNS when using alkylating agents, even those such as temozolomide which have good CNS penetration. Surprisingly, in this study increased median OS was also noted in patients with present MGMT expression [39]. This emphasizes that many questions regarding MGMT’s role in cancer biology have yet to be answered. Another study looking at MGMT expression and promoter methylation in brain metastases from solid tumors found the highest incidence (46.5%) of promoter methylation in lung cancers [40]. Ethnicity of the patients was not correlated with MGMT expression or promoter methylation. There are currently no published studies comparing these factors between C and AA.
Erlotinib and temozolomide have been used in combination in adults and children with primary CNS tumors as well as solid tumors elsewhere in the body [41-44]. We were able to demonstrate in patients with progressive brain metastases from lung cancer median OS 28 weeks, median TTP in CNS 14 weeks, and median TTP systemically 7.5 weeks. All patients on this regimen had SD in the CNS with some maintaining SD radiographically for prolonged intervals with stability clinically as well. A palliative regimen of erlotinib and temozolomide could be considered in patients in this setting. Correlative studies looking at EGFR mutations and MGMT methylation status could help shed light on who the likeliest responders to such a regimen would be.
Acknowledgments
Informed consent was obtained from all patients in the study.
This work was supported by the following grants: The Ben & Catherine Ivy Foundation (RVL); NIH/National Cancer Institute (5R01CA125541-03, 3R01CA125541-03S109, 5R01CA129501-02, 3R01CA129501-02S109, 5P01HL058064-140009), V-Foundation (Guy Gelded Memorial Foundation), Kate McMullen Foundation, Respiratory Health Association of Chicago, and Mesothelioma Applied Research Foundation (Jeffrey P. Hayes Memorial Grant) (RS).
| References | ▴Top |
- Ranjan T, Abrey LE. Current management of metastatic brain disease. Neurotherapeutics. 2009;6(3):598-603.
pubmed doi - Aizawa S, Fukushima T. A statistical analysis of computerized pathologic autopsy data in Japan from 1974 through 1993. The Information Management Committee of the Japanese Society of Pathology. Pathol Int. 1997;47(2-3):126-146.
pubmed doi - Shaffrey ME, Mut M, Asher AL, Burri SH, Chahlavi A, Chang SM, Farace E, et al. Brain metastases. Curr Probl Surg. 2004;41(8):665-741.
pubmed doi - Sanchez de Cos J, Sojo Gonzalez MA, Montero MV, Perez Calvo MC, Vicente MJ, Valle MH. Non-small cell lung cancer and silent brain metastasis. Survival and prognostic factors. Lung Cancer. 2009;63(1):140-145.
pubmed doi - Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, Bhatt A, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655-661.
pubmed doi - Sze G, Johnson C, Kawamura Y, Goldberg SN, Lange R, Friedland RJ, Wolf RJ. Comparison of single- and triple-dose contrast material in the MR screening of brain metastases. AJNR Am J Neuroradiol. 1998;19(5):821-828.
pubmed - Sadikov E, Bezjak A, Yi QL, Wells W, Dawson L, Millar BA, Laperriere N. Value of whole brain re-irradiation for brain metastases—single centre experience. Clin Oncol (R Coll Radiol). 2007;19(7):532-538.
pubmed doi - Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, Nishiwaki Y, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003;21(12):2237-2246.
pubmed doi - Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123-132.
pubmed doi - Abrey LE, Olson JD, Raizer JJ, Mack M, Rodavitch A, Boutros DY, Malkin MG. A phase II trial of temozolomide for patients with recurrent or progressive brain metastases. J Neurooncol. 2001;53(3):259-265.
pubmed doi - Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745-751.
pubmed doi - Ammirati M, Cobbs CS, Linskey ME, Paleologos NA, Ryken TC, Burri SH, Asher AL, et al. The role of retreatment in the management of recurrent/progressive brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):85-96.
pubmed doi - Nicholas MK, Lukas R. Immunologic privilege and the brain. In: Arnason BG, editor. Neuroimmune Biology. Volume 9. London: Elsevier, 2010: 169-181.
- Broniscer A, Panetta JC, O'Shaughnessy M, Fraga C, Bai F, Krasin MJ, Gajjar A, et al. Plasma and cerebrospinal fluid pharmacokinetics of erlotinib and its active metabolite OSI-420. Clin Cancer Res. 2007;13(5):1511-1515.
pubmed doi - Buie LW, Lindley C, Shih T, et al. Plasma pharmacokinetics and cerebrospinal fluid concentrations of erlotinib in high-grade gliomas: a novel, phase I, dose escalation study. [Abstract] J Clin Oncol. 2007;25(18S):2054.
- Raizer JJ, Abrey LE, Lassman AB, Chang SM, Lamborn KR, Kuhn JG, Yung WK, et al. A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro Oncol. 2010;12(1):95-103.
pubmed doi - Weber B, Winterdahl M, Memon A, Sorensen BS, Keiding S, Sorensen L, Nexo E, et al. Erlotinib accumulation in brain metastases from non-small cell lung cancer: visualization by positron emission tomography in a patient harboring a mutation in the epidermal growth factor receptor. J Thorac Oncol. 2011;6(7):1287-1289.
pubmed doi - Popat S, Hughes S, Papadopoulos P, Wilkins A, Moore S, Priest K, Meehan L, et al. Recurrent responses to non-small cell lung cancer brain metastases with erlotinib. Lung Cancer. 2007;56(1):135-137.
pubmed doi - Gounant V, Wislez M, Poulot V, Khalil A, Lavole A, Cadranel J, Milleron B. Subsequent brain metastasis responses to epidermal growth factor receptor tyrosine kinase inhibitors in a patient with non-small-cell lung cancer. Lung Cancer. 2007;58(3):425-428.
pubmed doi - Choong NW, Dietrich S, Seiwert TY, Tretiakova MS, Nallasura V, Davies GC, Lipkowitz S, et al. Gefitinib response of erlotinib-refractory lung cancer involving meninges—role of EGFR mutation. Nat Clin Pract Oncol. 2006;3(1):50-57; quiz 51 p following 57.
pubmed - Dhruva N, Socinski MA. Carcinomatous meningitis in non-small-cell lung cancer: response to high-dose erlotinib. J Clin Oncol. 2009;27(22):e31-32.
pubmed doi - Wagner M, Besse B, Balleyguier C, Soria JC. Leptomeningeal and medullary response to second-line erlotinib in lung adenocarcinoma. J Thorac Oncol. 2008;3(6):677-679.
pubmed doi - Namba Y, Kijima T, Yokota S, Niinaka M, Kawamura S, Iwasaki T, Takeda Y, et al. Gefitinib in patients with brain metastases from non-small-cell lung cancer: review of 15 clinical cases. Clin Lung Cancer. 2004;6(2):123-128.
pubmed doi - Hotta K, Kiura K, Ueoka H, Tabata M, Fujiwara K, Kozuki T, Okada T, et al. Effect of gefitinib ('Iressa', ZD1839) on brain metastases in patients with advanced non-small-cell lung cancer. Lung Cancer. 2004;46(2):255-261.
pubmed doi - Eichler AF, Kahle KT, Wang DL, Joshi VA, Willers H, Engelman JA, Lynch TJ, et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol. 2010;12(11):1193-1199.
pubmed doi - Ma S, Xu Y, Deng Q, Yu X. Treatment of brain metastasis from non-small cell lung cancer with whole brain radiotherapy and Gefitinib in a Chinese population. Lung Cancer. 2009;65(2):198-203.
pubmed doi - Kim JE, Lee DH, Choi Y, Yoon DH, Kim SW, Suh C, Lee JS. Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer. 2009;65(3):351-354.
pubmed doi - Ceresoli GL, Cappuzzo F, Gregorc V, Bartolini S, Crino L, Villa E. Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann Oncol. 2004;15(7):1042-1047.
pubmed doi - Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466.
pubmed doi - Ostermann S, Csajka C, Buclin T, Leyvraz S, Lejeune F, Decosterd LA, Stupp R. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004;10(11):3728-3736.
pubmed doi - Ebert BL, Niemierko E, Shaffer K, Salgia R. Use of temozolomide with other cytotoxic chemotherapy in the treatment of patients with recurrent brain metastases from lung cancer. Oncologist. 2003;8(1):69-75.
pubmed doi - Verger E, Gil M, Yaya R, Vinolas N, Villa S, Pujol T, Quinto L, et al. Temozolomide and concomitant whole brain radiotherapy in patients with brain metastases: a phase II randomized trial. Int J Radiat Oncol Biol Phys. 2005;61(1):185-191.
pubmed doi - Antonadou D, Paraskevaidis M, Sarris G, Coliarakis N, Economou I, Karageorgis P, Throuvalas N. Phase II randomized trial of temozolomide and concurrent radiotherapy in patients with brain metastases. J Clin Oncol. 2002;20(17):3644-3650.
pubmed doi - Mehta MP, Paleologos NA, Mikkelsen T, Robinson PD, Ammirati M, Andrews DW, Asher AL, et al. The role of chemotherapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):71-83.
pubmed doi - Lind JS, Lagerwaard FJ, Smit EF, Senan S. Phase I study of concurrent whole brain radiotherapy and erlotinib for multiple brain metastases from non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;74(5):1391-1396.
pubmed doi - Raizer JJ, Abrey LE, Lassman AB, Chang SM, Lamborn KR, Kuhn JG, Yung WK, et al. A phase I trial of erlotinib in patients with nonprogressive glioblastoma multiforme postradiation therapy, and recurrent malignant gliomas and meningiomas. Neuro Oncol. 2010;12(1):87-94.
pubmed doi - Ahmed SM, Salgia R. Epidermal growth factor receptor mutations and susceptibility to targeted therapy in lung cancer. Respirology. 2006;11(6):687-692.
pubmed doi - Krishnaswamy S, Kanteti R, Duke-Cohan JS, Loganathan S, Liu W, Ma PC, Sattler M, et al. Ethnic differences and functional analysis of MET mutations in lung cancer. Clin Cancer Res. 2009;15(18):5714-5723.
pubmed doi - Wu PF, Kuo KT, Kuo LT, Lin YT, Lee WC, Lu YS, Yang CH, et al. O(6)-Methylguanine-DNA methyltransferase expression and prognostic value in brain metastases of lung cancers. Lung Cancer. 2010;68(3):484-490.
pubmed doi - Ingold B, Schraml P, Heppner FL, Moch H. Homogeneous MGMT immunoreactivity correlates with an unmethylated MGMT promoter status in brain metastases of various solid tumors. PLoS One. 2009;4(3):e4775.
pubmed doi - Brown PD, Krishnan S, Sarkaria JN, Wu W, Jaeckle KA, Uhm JH, Geoffroy FJ, et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol. 2008;26(34):5603-5609.
pubmed doi - Prados MD, Chang SM, Butowski N, DeBoer R, Parvataneni R, Carliner H, Kabuubi P, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol. 2009;27(4):579-584.
pubmed doi - Peereboom DM, Shepard DR, Ahluwalia MS, Brewer CJ, Agarwal N, Stevens GH, Suh JH, et al. Phase II trial of erlotinib with temozolomide and radiation in patients with newly diagnosed glioblastoma multiforme. J Neurooncol. 2010;98(1):93-99.
pubmed doi - Jakacki RI, Hamilton M, Gilbertson RJ, Blaney SM, Tersak J, Krailo MD, Ingle AM, et al. Pediatric phase I and pharmacokinetic study of erlotinib followed by the combination of erlotinib and temozolomide: a Children's Oncology Group Phase I Consortium Study. J Clin Oncol. 2008;26(30):4921-4927.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.
