| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website https://www.neurores.org |
Original Article
Volume 12, Number 3, October 2022, pages 121-127
The Impact of Atrial Fibrillation on the Outcome of Ischemic Stroke Treated With Thrombolysis or Endovascular Therapy
Reza Bavarsad Shahripoura, Datis Azarpazhoohb, Benjamin Shifflettc, Sima Osoulid, Brett C. Meyere, Dawn Matherne Meyerf, g
aUCSD Neurology Department, Comprehensive Stroke Center, San Diego, CA, USA
bDepartment of Medical Sciences, University of Western Ontario, London, ON, Canada
cDepartment of Neurosciences, University of California, San Diego, La Jolla, CA, USA
dDepartment of Neurology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
eDepartment of Neurosciences, UCSD Enterprise Telemedicine, San Diego, CA, USA
fDepartment of Neurosciences, School of Medicine, UC San Diego, La Jolla, CA, USA
gCorresponding Author: Dawn Matherne Meyer, Department of Neurosciences, UC San Diego Health Science Center, La Jolla, CA, USA
Manuscript submitted August 27, 2022, accepted October 12, 2022, published online October 22, 2022
Short title: Atrial Fibrillation and Stroke Outcomes
doi: https://doi.org/10.14740/jnr738
| Abstract | ▴Top |
Background: The purpose of this study was to assess the impact of atrial fibrillation (AF) on stroke outcome in acute ischemic stroke (AIS) treated with thrombolysis or endovascular therapy (EVT).
Method: Consecutive AIS treated at five stroke centers over 15 years was evaluated. Using multiple logistic regressions, we compared adjusted odds ratio (OR) with 95% confidence interval (CI) of symptomatic intracranial hemorrhage (sICH), thrombolysis in cerebral infarction (TICI), poor discharge disposition (hospice/skilled nursing facility), in-hospital mortality, and disability (modified Rankin Scale (mRS)) and death at 90 days. Patients were classified according to AF and treatment plans, including tissue plasminogen activator (tPA) or EVT.
Results: We identified 720 patients treated in the study period (196 +AF, aged 78.2 ± 12.5 years and 524 -AF, aged 66.9 ± 15.2 years). In adjusted logistic regression, there was no difference in the rate of sICH (OR: 1.18, 95% CI: 0.44 - 3.17), TICI (OR: 1.19, 95% CI: 0.29 - 4.84), poor discharge disposition to hospice/skilled nursing facility (OR: 0.96, 95% CI: 0.56 - 1.63) or in-hospital mortality (OR: 0.95, 95% CI: 0.46 - 1.97). There was no significant difference in a 90-day mRS in those with and without AF. Likewise, there were no between group differences in sICH, discharge outcome, or 90-day mRS in the six groups based on the presence of AF and treatment plans (tPA, EVT, or both).
Conclusions: AF did not significantly impact sICH, discharge outcome, or 90-day mRS in AIS patients treated with thrombolysis, EVT, or both. Age and baseline National Institutes of Health Stroke Scale (NIHSS) were significant predictors of outcome in this sample.
Keywords: Ischemic stroke; Atrial fibrillation; Endovascular treatment; Tissue plasminogen activator; Symptomatic intracranial hemorrhage
| Introduction | ▴Top |
Atrial fibrillation (AF) is a major risk factor of vascular disease with a 4-5-fold increased risk of acute ischemic stroke (AIS) [1]. The attributable risk of stroke to AF in patients aged 50 - 59 years is 1.5%, increasing to nearly 25% for those aged ≥ 80 years [1, 2]. Despite the significant role of AF in stroke risk, the impact of AF on stroke outcome of those receiving tissue plasminogen activator (tPA) and/or endovascular therapy (EVT) is still unclear. In one study of AIS patients with severe stroke, those with AF had a higher proportion of favorable 90-day outcomes than those without AF (odds ratio (OR): 5.80, 95% confidence interval (CI): 1.63 - 20.68). This was not observed in patients with mild stroke [3]. Other studies have shown that AF is an independent predictor of no recanalization after thrombolysis [4]. Tu et al suggested that the worse stroke outcome in AF may be explained by more severe hypoperfusion, infarct growth, and hemorrhagic transformation [5]. However, a study using the Virtual International Stroke Trials Archive (VISTA; n = 7,091 patients) database did not find a significant association between AF and stroke outcome [6]. Previous embolic ischemic stroke animal models, which combined recombinant tPA (r-tPA) with an antiplatelet agent or statin revealed decrease in worse outcomes and increase in treatment window to 6 h [7, 8].
The role of AF in stroke outcome and response to acute therapy is still unclear. This study aimed to assess the impact of AF on short-term stroke outcome in AIS patients treated with tPA or EVT, or both.
| Materials and Methods | ▴Top |
Study population
We retrospectively reviewed prospectively collected data from an IRB-approved registry (USCD; IRB# 800900). We included all consecutive stroke code activations between January 2004 and June 2020 from five acute stroke centers (three comprehensive stroke centers and two primary stroke centers) in San Diego, California, that a single provider group staff. Patients were included if they were treated with tPA, EVT, or both within 24 h of stroke onset based on current stroke treatment guidelines. Cases with AF (AF+) were identified based on the previous recorded history or patient/family discussion at any time in the past or diagnosed during hospitalization. Patients were stratified into six groups depending on their history of AF and whether or not they received treatment (tPA, EVT, or both) (Fig. 1).
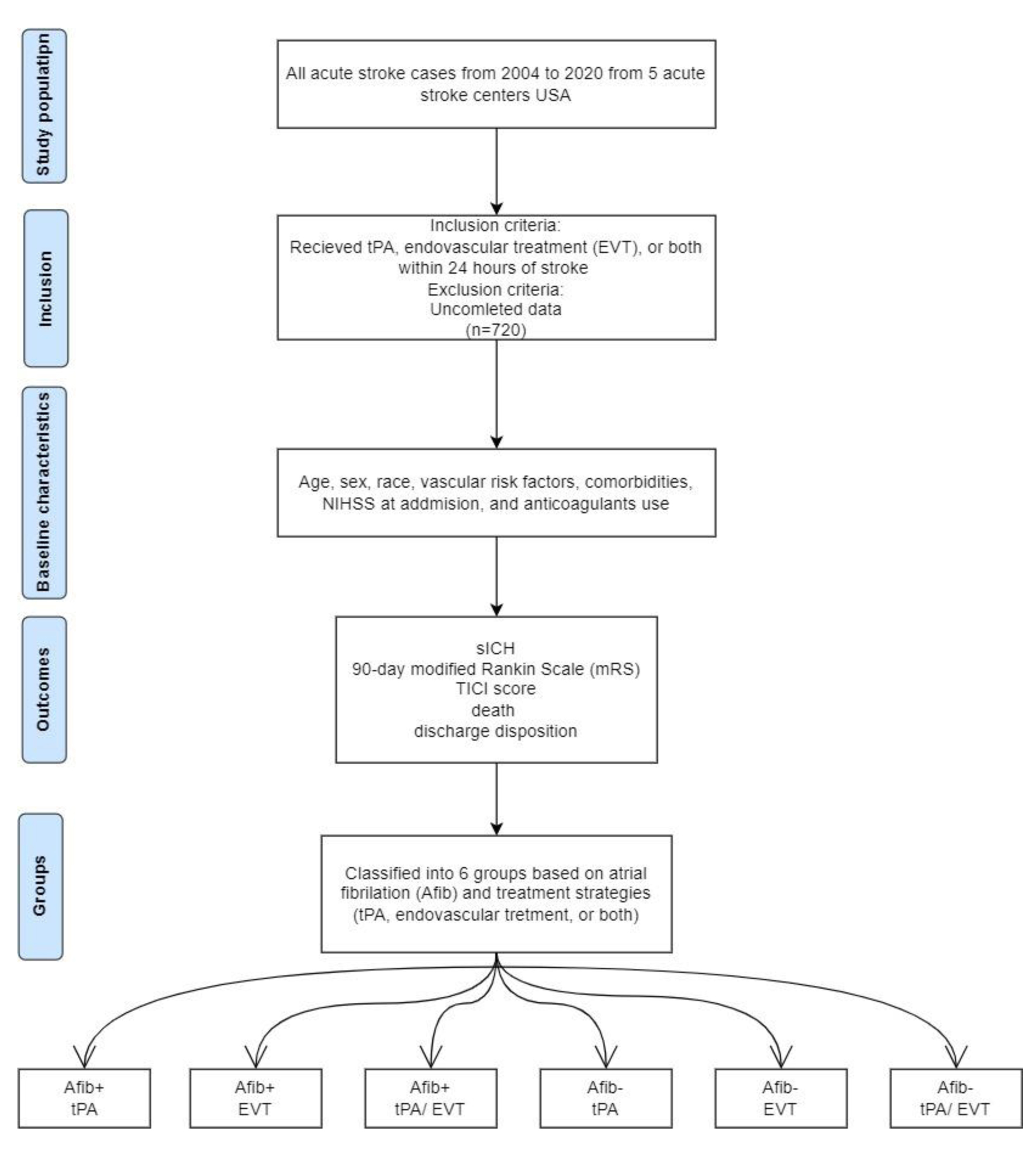 Click for large image | Figure 1. The algorithm of study design. |
Outcome measures
Baseline characteristics were prospectively collected. Socio-demographic, baseline vascular risk factors, comorbidities, and radiological information were assessed. The National Institutes of Health Stroke Scale (NIHSS) measured admission severity at admission [9]. We defined symptomatic intracranial hemorrhage (sICH) based on the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) criteria [10]. Contrast extravasation versus sICH was determined based on clinical exam and magnetic resonance imaging findings. We defined discharge disposition as good (discharge to home or acute rehabilitation facility and poor discharge) vs. poor (discharge to a skilled nursing facility, hospice, or in-hospital death). Stroke outcome was assessed by a 90-day modified Rankin Scale (mRS) with a good outcome defined as mRS 0 - 2 [11]. Thrombolysis in cerebral infarction (TICI) score was used to assess successful revascularization after EVT [12]. Poor recanalization was defined as TICI score < 2b (2a, 1, and 0) [13].
Statistical analysis
Baseline demographics were compared between groups using Pearson’s Chi-squared test for categorical variables and analysis of variance (ANOVA) for continuous variables. We used Brown-Mood median test to compare the median baseline NIHSS score by the group. To compare any differences in functional outcome (90-day mRS) between groups, we performed a pairwise Wilcoxon rank test followed by adjusted multinomial regression. We used multiple logistic regressions to compare the adjusted OR with 95% CI of 90-day mRS, discharge disposition, and sICH between baseline AF- and AF+ subjects. A similar analysis was performed among six groups of patients according to the presence or absence of AF and treatment plans (tPA, EVT, or both) and within the six groups (AF + receiving only thrombolysis as the reference group). Data were adjusted for baseline NIHSS, age, sex, baseline systolic blood pressure, pre-stroke mRS, smoking status, glucose, anticoagulant use, and congestive heart failure (CHF) at admission. All analyses used an alpha of 0.05, and a P-value ≤ 0.05 was considered significant.
| Results | ▴Top |
We identified 720 AIS patients who received tPA or EVT or both, including 196 AF+ (55.6% women) and 524 AF- (43.3% women). Those with AF were older (78.2 ± 12.5 vs. 66.9 ± 15.2; P < 0.0001) and had a higher rate of CHF (21.4% vs. 9.5%; P = 0.0001) and a lower rate of smoking (3.1% vs. 16%; P < 0.0001). In addition, AF+ had a more severe stroke at admission as measured by NIHSS > 5 (77.4% vs. 64.4%; P = 0.0014) (Table 1). A majority of AF patients were white (n = 583; 28.5%), followed by Hispanic/Latino (n = 146; 26%) and African Americans (n = 60; 3.7%). Details of groups based on their treatment plans are summarized in Tables 2 and 3.
 Click to view | Table 1. Demographic Data, Vascular Risk Factors and Baseline Assessments: Patient Classification According to Those With and Without Atrial Fibrillation |
 Click to view | Table 2. A Comparison Between the Rate of Successful Recanalization and Hemorrhagic Transformation in Cases With and Without Atrial Fibrillation: The Result of Adjusted Logistics Regression |
 Click to view | Table 3. A Comparison Between Discharge Disposition and Poststroke Disability in Cases With and Without Atrial Fibrillation: The Result of Adjusted Logistics Regression |
Outcome variables
Twenty-four cases had sICH (nine cases with AF and 15 without AF). The rate of sICH did not have a significant difference between cases with and without AF (OR: 1.18, 95% CI: 0.44 - 3.17, P = 0.74). We compared TICI scores in those treated with EVT. Six cases with AF and 11 without AF had TICI < 2b (P = 0.89). In adjusted regression analysis, the rate of TICI < 2b was not significantly different among patients with and without AF (OR: 1.19, CI: 0.29 - 4.84, P = 0.81) (Table 2). We classified poor short-term outcomes into in-hospital death (18 (9.2%) of AF+ vs. 35 (6.7%) of AF-; P = 0.3), discharge to hospice or skilled nursing facility (47 (14.1%) of AF+ vs. 74 (23.9%) of AF-; P < 0.0001) and composite rate of hospital death with discharge to hospice and skilled nursing facility (65 (33.2%) of AF+ vs. 109 (20.8%) of AF-; P < 0.0001). In adjusted analysis, there was no significant difference in the rate of in-hospital death (OR: 0.95, 95% CI: 0.46 - 1.97, P = 0.89), discharge to hospice or skilled nursing facility only (OR: 0.96, 95% CI: 0.56 - 1.63, P = 0.87) and a combined rate of death and hospice/skilled nursing facility (OR: 1, 95% CI: 0.63 - 1.59, P = 0.99) (Table 3).
All cases discharged from the hospital were success assessed at 90 days post-stroke (n = 667). Three models were run for the 90-day mRS analysis (score of 0 - 2 was a good outcome), one with poor outcome as both moderate and severe disability as well as death (mRS score of 3 - 6; OR: 0.73, 95% CI: 0.48 - 1.11, P = 0.14), moderate and severe disability only (score of 3 - 5; OR: 0.75, 95% CI: 0.47 - 1.20, P = 0.23) and death only (score of 6; OR: 0.67, 95% CI: 0.37 - 1.21, P = 0.19). We did not find any significant difference based on the presence of AF. Results for the three models are shown in Table 3. Likewise, in the subgroup analysis based on stroke etiology and treatment plan as compared to the reference group, there were no significant differences in the adjusted odds of TICI, sICH, discharge disposition (including hospice/skilled nursing facility, and composite rate of discharge to hospice/skilled nursing facility and death) and post-stroke disability in cases with AF (Supplementary Materials 1, 2 and 3, www.neurores.org).
| Discussion | ▴Top |
Our study has important clinical implications. Among 720 cases with AIS, AF was not a determinant of poor outcome in patients treated with thrombolytic therapy, EVT, or both. In adjusted analysis, stroke disability, discharge disposition, symptomatic hemorrhage, and TICI score were not different according to AF etiology and treatment arms.
The overall rate of AF has increased significantly with more cases of stroke attributed to AF [14]. Despite the importance of AF in stroke, available data about the role of AF in stroke outcomes are controversial. A meta-analysis by Yue et al emphasized that AF may increase the risk of adverse outcomes for AIS undergoing thrombolysis due to an increase in the rate of sICH and death [15]. Another meta-analysis of 18 studies reported worse outcomes in AF versus non-AF cases of ischemic stroke treated with thrombolysis and higher incidence of sICH in patients with AF treated with thrombolysis compared to the other therapies [16]. On the other hand, Tong et al reported no significant differences in the mRS score, successful recanalization, sICH and mortality between patients with and without AF after EVT [17]. In contrast, Zhang et al found that patients with stroke and AF may benefit from tPA in terms of 90-day follow-up [18]. A nationwide cohort study in Taiwan reported although a higher risk of ICH according to the National Institute of Neurological Disorders and Stroke standard (NINDS) in the AF group treated with alteplase but no differences in sICH according to SITS-MOST standards, favorable 90-day outcome, and mortality in comparison with the non-AF group [19]. Among 143 AIS patients who received intravenous thrombolysis, AF was associated with a favorable 90-day outcome in patients with severe stroke at baseline (NIHSS > 10). However, this association did not exist in patients with mild stroke and AF [20]. Our study is novel as we have added information regarding thrombolysis or EVT. In our study, AF was not associated with poor outcomes as defined by discharge disposition, in-hospital death, and a 3-month post-stroke disability. While some studies found a higher rate of hemorrhagic transformation after thrombolysis [21], we did not find this trend in our cases.
There is also controversy about the rate of recanalization after thrombolysis in AF patients. Some studies have found similar [11], lower [4], or even higher recanalization rates in the AF population compared to non-AF [22]. Differences in clot organization and collagen composite can affect responses to medical or mechanical reperfusion therapy [18, 23]. In our cases, AF did not impact the degree of recanalization as evidenced by the TICI score after EVT.
It is important to assess the effect of age on all AF studies. AF is more prevalent in older adults, with a steady increase in its prevalence by aging from 0.12% to 0.16% in those aged < 49 years to 10-17% in those aged > 80 years [24]. In our study, cases with AF were significantly older than those without AF. In the VISTA study, 29% of the variability of outcome in AF patients was accounted for by age and baseline NIHSS. After adjustment for these variables, AF was not associated with the 90-day outcomes [6]. Likewise, Saposnik et al found a correlation between AF with older age and higher baseline NIHSS. Patients with AF had higher mortality and rates of sICH [25]. Another study reported a higher rate of unfavorable outcomes in patients with AF aged ≥ 80 versus the rest after EVT in AIS [26]. Our study is important as we adjusted our data not only for age and sex but also for baseline NIHSS and pre-stroke mRS. Our patients had a more severe stroke (according to their baseline NIHSS), comorbidities (such as CHF), and pre-stroke disability (according to baseline mRS). We did not observe a significant difference in cases with AF treated with thrombolysis, EVT, or both compared to other groups. Therefore, some previous reports of worse outcomes in patients with AF in clinical practice may be due to the aging effect, baseline disability, limitation for thrombolytic therapy, and more severe strokes in cases with AF.
Our study has some limitations. First, this is a retrospective study that may have an inherent bias due to the convenience sampling method. Second, we did not have access to the type of AF (i.e., paroxysmal or chronic) that can influence the outcomes. Third, we followed our case for 3 months, and we cannot comment on the long-term outcome of our cases. Fourth, we did not measure the effect of the door-to-needle times in thrombolysis and door-to-device times in endovascular treatment on patients’ outcomes. Major strengths of our study include access to detailed clinical and radiological information on cases with AF treated with tPA, EVT, or both. In the next phase of the study, we are planning to evaluate imaging variables, including infarct size, reperfusion rate, infarct core growth rate, and collaterals, to identify differences in cases with AF as compared to other groups. We will also try to increase our sample size to perform a detailed analysis based on different subgroups, particularly for those with AF and EVT.
Conclusion
In this study, AF did not significantly impact the outcome in AIS patients treated with thrombolysis, EVT, or both. Patients with AF are older and have had more severe strokes and baseline disability. A close clinical follow-up during their admissions. Further studies with larger sample sizes that include rigorous radiographic, clot morphology, and collateral flow factors are needed to optimize the acute treatment of stroke patients with AF.
Learning points
AF is a leading cause of ischemic stroke.
Those with AF are older and have more disability/comorbidities at baseline.
Differences in the clinical outcome of AF cases are likely due to stroke severity, older age, and comorbidities rather than AF alone.
| Supplementary Material | ▴Top |
Suppl 1. Demographic data, vascular risk factors and baseline assessments: patient classification according to those with and without atrial fibrillation and treatment plans.
Suppl 2. A comparison between the rate of successful recanalization and hemorrhagic transformation in cases with and without atrial fibrillation according to treatment plans: the result of adjusted logistics regression.
Suppl 3. A comparison between discharge disposition and poststroke disability in cases with and without atrial fibrillation according to treatment plans: the result of adjusted logistics regression.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Reza Bavarsad Shahripour: study design, writing the first draft, and data analysis. Datis Azarpazhooh: writing the first draft, data analysis, and revision of the manuscript. Benjamin Shifflett: data analysis and revision of the paper. Sima Osouli: revision of the draft. Brett C. Meyer: data gathering and revision of the paper. Dawn Meyer: study design, revision of the final draft, and study supervisor.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
AF: atrial fibrillation; tPA: tissue plasminogen activator; EVT: endovascular therapy; AIS: acute ischemic stroke; VISTA: Virtual International Stroke Trials Archive; NIHSS: National Institutes of Health Stroke Scale; sICH: symptomatic intracranial hemorrhage; SITS-MOST: The Safe Implementation of Thrombolysis in Stroke-Monitoring Study; mRS: modified Rankin Scale; TICI: thrombolysis in cerebral infarction; OR: odds ratio; CI: confidence interval; CHF: congestive heart failure; NINDS: National Institute of Neurological Disorders and Stroke standard
| References | ▴Top |
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983-988.
doi pubmed - Hart RG, Pearce LA, Miller VT, Anderson DC, Rothrock JF, Albers GW, Nasco E. Cardioembolic vs. noncardioembolic strokes in atrial fibrillation: frequency and effect of antithrombotic agents in the stroke prevention in atrial fibrillation studies. Cerebrovasc Dis. 2000;10(1):39-43.
doi pubmed - Saposnik G, Gladstone D, Raptis R, Zhou L, Hart RG, Investigators of the Registry of the Canadian Stroke N, the Stroke Outcomes Research Canada Working G. Atrial fibrillation in ischemic stroke: predicting response to thrombolysis and clinical outcomes. Stroke. 2013;44(1):99-104.
doi pubmed - Kimura K, Iguchi Y, Yamashita S, Shibazaki K, Kobayashi K, Inoue T. Atrial fibrillation as an independent predictor for no early recanalization after IV-t-PA in acute ischemic stroke. J Neurol Sci. 2008;267(1-2):57-61.
doi pubmed - Tu HT, Campbell BC, Christensen S, Desmond PM, De Silva DA, Parsons MW, Churilov L, et al. Worse stroke outcome in atrial fibrillation is explained by more severe hypoperfusion, infarct growth, and hemorrhagic transformation. Int J Stroke. 2015;10(4):534-540.
doi pubmed - Frank B, Fulton R, Weimar C, Shuaib A, Lees KR, Collaborators V. Impact of atrial fibrillation on outcome in thrombolyzed patients with stroke: evidence from the Virtual International Stroke Trials Archive (VISTA). Stroke. 2012;43(7):1872-1877.
doi pubmed - Tan Z, Li X, Turner RC, Logsdon AF, Lucke-Wold B, DiPasquale K, Jeong SS, et al. Combination treatment of r-tPA and an optimized human apyrase reduces mortality rate and hemorrhagic transformation 6h after ischemic stroke in aged female rats. Eur J Pharmacol. 2014;738:368-373.
doi pubmed - Tan Z, Lucke-Wold BP, Logsdon AF, Turner RC, Tan C, Li X, Hongpaison J, et al. Bryostatin extends tPA time window to 6 h following middle cerebral artery occlusion in aged female rats. Eur J Pharmacol. 2015;764:404-412.
doi pubmed - National Institute of Neurological Disorders. Stroke rt, P. A. Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581-1587.
doi pubmed - Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, Hennerici MG, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369(9558):275-282.
doi - Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38(3):1091-1096.
doi pubmed - Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, Dillon W, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34(8):e109-137.
doi - Fugate JE, Klunder AM, Kallmes DF. What is meant by "TICI"? AJNR Am J Neuroradiol. 2013;34(9):1792-1797.
doi pubmed - Williams BA, Chamberlain AM, Blankenship JC, Hylek EM, Voyce S. Trends in atrial fibrillation incidence rates within an integrated health care delivery system, 2006 to 2018. JAMA Netw Open. 2020;3(8):e2014874.
doi pubmed - Yue R, Li D, Yu J, Li S, Ma Y, Huang S, Zeng Z, et al. Atrial fibrillation is associated with poor outcomes in thrombolyzed patients with acute ischemic stroke: a systematic review and meta-analysis. Medicine (Baltimore). 2016;95(10):e3054.
doi pubmed - Hu Y, Ji C. Efficacy and safety of thrombolysis for acute ischemic stroke with atrial fibrillation: a meta-analysis. BMC Neurol. 2021;21(1):66.
doi pubmed - Tong X, Li S, Liu W, Ren Z, Liu R, Jia B, Zhang X, et al. Endovascular treatment for acute ischemic stroke in patients with versus without atrial fibrillation: a matched-control study. BMC Neurol. 2021;21(1):377.
doi pubmed - Zhang JB, Ding ZY, Yang Y, Sun W, Hai F, Sui XN, Li XY, et al. Thrombolysis with alteplase for acute ischemic stroke patients with atrial fibrillation. Neurol Res. 2010;32(4):353-358.
doi pubmed - Lin SF, Chen CF, Hu HH, Ho BL, Chen CH, Chan L, Lin HJ, et al. Comparison of different dosages of alteplase in atrial fibrillation-related acute ischemic stroke after intravenous thrombolysis: a nationwide, multicenter, prospective cohort study in Taiwan. J Am Heart Assoc. 2022;11(3):e023032.
doi pubmed - Sung SF, Chen YW, Tseng MC, Ong CT, Lin HJ. Atrial fibrillation predicts good functional outcome following intravenous tissue plasminogen activator in patients with severe stroke. Clin Neurol Neurosurg. 2013;115(7):892-895.
doi pubmed - Dang H, Ge WQ, Zhou CF, Zhou CY. The Correlation between Atrial Fibrillation and Prognosis and Hemorrhagic Transformation. Eur Neurol. 2019;82(1-3):9-14.
doi pubmed - Molina CA, Alexandrov AV, Demchuk AM, Saqqur M, Uchino K, Alvarez-Sabin J, Investigators C. Improving the predictive accuracy of recanalization on stroke outcome in patients treated with tissue plasminogen activator. Stroke. 2004;35(1):151-156.
doi pubmed - Kimura K, Iguchi Y, Shibazaki K, Iwanaga T, Yamashita S, Aoki J. IV t-PA therapy in acute stroke patients with atrial fibrillation. J Neurol Sci. 2009;276(1-2):6-8.
doi pubmed - Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213-220.
doi pubmed - Saposnik G, Kapral MK, Liu Y, Hall R, O'Donnell M, Raptis S, Tu JV, et al. IScore: a risk score to predict death early after hospitalization for an acute ischemic stroke. Circulation. 2011;123(7):739-749.
doi pubmed - Jiao J, Liu S, Cui C, Cao Y, Jia Z, Liu H, Wang C, et al. Endovascular thrombectomy for acute ischemic stroke in elderly patients with atrial fibrillation. BMC Neurol. 2022;22(1):100.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.
