| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website https://www.neurores.org |
Original Article
Volume 12, Number 3, October 2022, pages 114-120
A Novel Radiographic and Clinical Assessment Scoring Tool for Seizure Risk Stratification in the Acute Phase of Cerebral Venous Sinus Thrombosis: A Systematic Review and Meta-Analysis
Stella Paka, d, e, Sahil Sardanab, Rudy Estessc, d, Nihita Manema, Tamer Abdelhaka
aNeurology Department, Albany Medical Center, Albany, NY 12208, USA
bMedicine Department, Dayanand Medical College, Civil Lines, Tagore Nagar, Ludhiana, Punjab 141001, India
cFamily Medicine Department, Kaiser Permanente Facility, Redwood City, CA 94063, USA
dThese authors contributed equally to this article.
eCorresponding Author: Stella Pak, Neurology Department, Albany Medical Center, Albany, NY 12208, USA
Manuscript submitted May 13, 2022, accepted July 13, 2022, published online August 8, 2022
Short title: A Novel RC Scoring Tool in Acute CVST
doi: https://doi.org/10.14740/jnr731
| Abstract | ▴Top |
Background: Nearly 40% of cerebral venous sinus thrombosis (CVST) cases experience a seizure. A plethora of problems may arise from seizures. Many of these are well recognized in literature as well as in clinical practice. These include the risk for acute respiratory failure, acute renal injury, demand ischemia of myocardium, aspiration pneumonia, and a variety of musculoskeletal injury. Given the lack of a validated tool to predict seizure in the acute phase of CVST, the use of prophylactic anti-epileptic drugs (AEDs) is controversial. A systematic review and meta-analysis of observational studies was conducted to identify risk factors to construct a clinical prediction tool for seizure in acute CVST.
Methods: Systematic review was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Observational studies that investigated the risk factors for seizure in acute CVST were retrieved from MEDLINE, EBSCO, Web of Science, and Pro-Quest. The summary odds ratios (ORs) were calculated from the pool of data under the random effects model. A point value of 1, 2, or 3 was assigned to each risk factor based on their β-coefficient in the predictive model. Discriminative ability of this model was evaluated on the receiver operating characteristic curve.
Results: Initial literature search revealed 1,046 articles discussing seizure as a complication of acute CVST. Through a robust systematic review process with two independent reviewers, 14 studies fully meeting the inclusion criteria were selected. Data elements extracted from the studies were analyzed and re-synthesized. Anatomical involvement of frontal lobe (OR: 4.85; 95% confidence interval (CI): 3.52 - 6.68), parietal lobe (OR: 2.52, 95% CI: 1.41 - 4.52), cortical vein thrombosis (OR: 3.16, 95% CI: 2.18 - 4.58), hemorrhagic venous ischemia (OR: 3.85; 95% CI: 3.20 - 4.64), and clinical presentation of motor deficit (OR: 3.07; 95% CI: 2.66 - 3.55) or confusion (OR: 2.15; 95% CI: 1.57 - 2.94) showed a strong association with increased risk for seizure in the setting of acute CVST. We developed a novel Radiographic and Clinical Assessment (RC) scoring system, consisting of the significant six risk factors. RC score yielded a calculated area under the curve of 0.89, with probabilities for seizure ranging from 40% with a score of 0 to 92% for score of 6.
Conclusions: RC scoring tool can be used to stratify the seizure risk based on radiographic findings and the clinical presentation in the acute phase of CVST. This predictive tool may be helpful in identifying patients whose seizure risk is high and can potentially further be used as a clinical decision support tool for prophylactic AED treatment.
Keywords: Cerebral venous sinus thrombosis; Seizure; Stroke
| Introduction | ▴Top |
Acute symptomatic seizure is a transient episode of abnormal excessive or synchronous neuronal activity occurring in close temporal association with a neurological or systemic insult [1]. A plethora of problems may arise from seizures. Many of these are well recognized in literature as well as in clinical practice. These include the risk for acute respiratory failure, acute renal injury, demand ischemia of myocardium, aspiration pneumonia, and a variety of musculoskeletal injury [2]. Acute seizure following stroke, in particular, has been associated with increased morbidity and mortality. The metabolic demand from hypersynchronous neuronal discharge serve to aggravate ischemic episode in the penumbra [3]. This metabolic crisis with the release of extracellular glutamate results in further elevation of intracranial pressure. Therefore, prophylactic use of anti-epileptic drugs (AEDs) in individuals at high risk for seizure may have a role in post-stroke neuroprotection [4]. Acute symptomatic seizure is a rare complication of stroke, with estimates ranging from 3% to 4% [5]. Current recommendations are against primary prophylaxis for post-stroke seizure in view of the low incidence of seizure and known adverse events of AEDs [6, 7].
One of the areas of concern is the potential for drug interaction of enzyme-inducing AEDs in stroke patients. For instance, carbamazepine, phenytoin phenobarbital, and primidone are known to lower therapeutic efficacy of statins. Valproic acid and phenytoin are notorious for its insulin resistance side effect. Furthermore, a vast majority of AEDs are associated with obesity, dyslipidemia, and chronic fatigue. Thus, the effects of AEDs on vascular risk factors in stroke patients makes a trade-off analysis of AEDs for stroke critical [8]. Phenytoin and benzodiazepine class of AEDs are also shown to suppress the neural plasticity associated with cognitive and behavioral rehabilitation following stroke [9, 10]. When AEDs is given for a short-term prophylaxis, a continued coordination and monitoring is essential for safe discontinuation. Otherwise, anti-epileptic therapy can be perpetuated indefinitely, which can cause unnecessary financial and socio-psychological burden [11].
Nevertheless, the American Heart Association Stroke Council recommends judicious use of AEDs as primary prophylaxis for stroke patients at high risk for seizure [6]. Cerebral venous sinus thrombosis (CVST) is an uncommon type of strokes, representing less than 1% of all strokes [12, 13]. Approximately 40% of CVST patients experience acute symptomatic seizure [14]. CVST is a cause of stroke in which the venous conduits within the brain become thrombosed, resulting in venous flow obstruction [15]. Reported incidence of CVST is about seven cases per million adults per year. Nearly 75% of the CVST cases occur in females [16]. Although a rarer cause of stroke, accounting for about roughly less than 1% of all strokes, there are risk factors that have been well documented in the literature. Risks including but not limited to pregnancy/puerperium, use of oral contraceptives, thrombophilia, malignancy, smoking, and infection have all been associated with CVST [17].
Often, the initial presenting symptom is a headache, but there are many other reported clinical presentations of CVST as the clinical manifestations of CVST can be variable. Symptoms include, motor deficits, sensory deficits, nausea, vomiting, confusion, agitation, gait disturbance, cranial nerve findings, visual complaints, seizures, and coma [15]. This variable presentation of CVST creates an essential need for radiographic imaging. Imaging modalities such computed tomography, magnetic resonance (MR) imaging (MRI), angiography, venography, and MR venography can confirm a diagnosis of CVST [16]. Previously there had been poor prognosis associated with CVST, however with the advancement of neuroimaging and treatment interventions, CVST has been better identified and treated. Treatment guidelines for CVST center upon the rapid administration of antithrombotic therapy, close monitoring for and management of increased intracranial pressure, therapy for any associated psychomotor agitation, analgesic treatment for pain, antibiotics in the setting of infection, and treatment of seizures [18].
Given the lack of a validated tool to predict seizure in the acute phase of CVST, the use of prophylactic AEDs is controversial. Considering that there is a high incidence of seizures, the question regarding whether to treat seizures prophylactically in this setting is a relevant one. Literature surrounding this topic does not yield sufficient recommendations regarding the initiation of prophylactic treatment, duration of treatment, and how these clinical decisions impact prognosis and risk of recurrence [19]. In this study, we sought to develop a clinically feasible tool to help predict the risk of acute symptomatic seizure in patients with CVST in the acute setting. Our hope is that this tool can serve to aid clinicians and guide in management and treatment decisions.
| Materials and Methods | ▴Top |
Systematic review
Systematic review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline from 2009. First, studies were identified via query of MEDLINE, EBSCO, Web of Science, and Pro-Quest databases with following key words: “cerebral venous thrombosis,” “seizure,” and “cerebral vein and dural sinus thrombosis.” The trial query period was completed on February 25, 2022. Specific search terms used in each database are described here (Supplementary Material 1, www.neurores.org). Two-stage screening process with Covidence was used to remove duplicates. Reference lists of included articles were also investigated for the identification of relevant articles. The inclusion criteria included the following: 1) prospective or retrospective observational study; 2) studies reporting potential risk factors for seizure in the acute phase of CVST; and 3) studies with sufficient information to estimate the odds ratio (OR) and 95% confidence intervals (CIs). The exclusion criteria were: 1) editorial, letter to the editor, or case report; 2) abstracts or posters from conferences; 3) use of ambiguous exclusion criteria; 4) insufficient details on data collection methodology; 5) unpublished studies; 6) studies with the number of patients less than 30; and 7) studies on neonatal patients. Unpublished studies were excluded from our analysis due to difficulties in assessing their internal validity and reliability. We included all articles in which sufficient data were presented to allow the assessment of potential risk factors for seizure in acute CVST. Two reviewers independently performed study selection based on the inclusion and exclusion criteria (Fig. 1). Kappa statistics were calculated to assess the agreement between reviewers for relevance of study selection. Disagreements were resolved at a meeting between reviewers before data extraction. Data extraction was also conducted by two reviewers. A third reviewer compared the data extraction forms to ensure accuracy. Disagreements were resolved by discussion among the three reviewers.
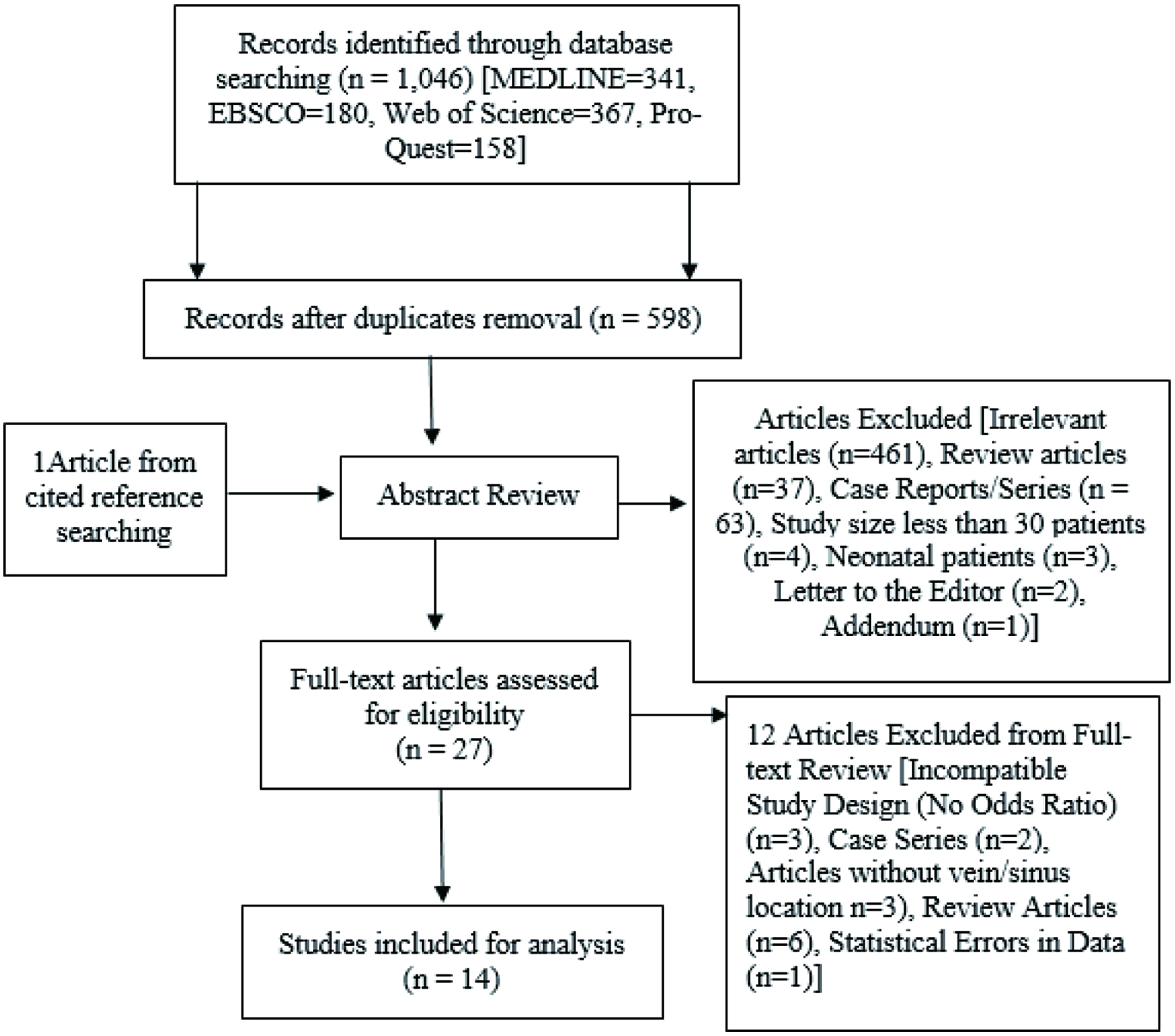 Click for large image | Figure 1. Flow diagram of search process and study selection. |
Statistical analysis
Data were entered into the Cochrane Review Manager software (RevMan 5.0) and analyzed using multivariate regression analysis. Random-effects model was used for meta-analyses of all variables. I2 determination was used to measure the heterogeneity. I2 index below 50% was considered indicative of no significant heterogeneity. ORs with 95% CI were calculated by the random-effects model for seizure occurrence between CVST patients with and without each risk factor studied. Potential risk factors are involvement of frontal lobe, parietal lobe, temporal lobe, occipital lobe, involvement of cortical vein, transverse (lateral) sinus, straight sinus, sigmoid sinus, deep or cerebral vein, presence of non-hemorrhagic or hemorrhagic ischemia on MRI, clinical finding of headache, confusion, aphasia, motor deficit, papilledema, sensory disturbance, nausea, or vomiting. A point value of 1, 2, or 3 was assigned to each risk factor based on their β-coefficient in the predictive model. We assessed the discriminative ability of this novel prediction score using the receiver operating characteristic curve.
This study was exempted from the institutional review board (IRB) approval (IRB case number: R_3pihWhik52WEBVq). The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
| Results | ▴Top |
A total of 14 studies with 4,414 patients were included for quantitative analyzation for the seizure risk factors in the acute phase of CVST [20-33]. Nine studies were included in the imaging studies, involving 563 patients. The Kappa statistic measuring agreement between the two reviewers at the title/abstract stage and the full-text stage were 0.7 and 0.9, respectively. This concordance coefficient suggests strong agreement between the reviewers.
As shown in Table 1, ORs were calculated by the random-effects model for seizure occurrence between CVST patients with and without each risk factor studied. The ORs and 95% CI for potential risk factors were as the following: frontal lobe (OR = 4.85, 95% CI = 3.52 - 6.68), parietal lobe (OR = 2.52, 95% CI = 1.41 - 4.52), temporal lobe (OR = 1.58, 95% CI = 0.60 - 4.14), occipital lobe (OR = 0.99, 95% CI 0.62 - 1.59), superficial sagittal sinus (OR = 1.97, 95% CI = 1.17 - 3.30), cortical vein (OR = 3.16, 95% CI = 2.18 - 4.58), transverse (lateral) sinus (OR = 0.60, 95% CI = 0.50 - 0.73), straight sinus (OR = 0.63, 95% CI = 0.40 - 0.90), sigmoid sinus (OR = 1.11, 95% CI = 0.74 - 1.66), deep cerebral vein (OR = 0.39, 95% CI = 0.07 - 2.07), hemorrhagic ischemia (OR = 3.85, 95% CI = 3.20 - 4.64), non-hemorrhagic ischemia (OR = 1.49, 95% CI = 1.13 - 1.98), headache (OR = 0.35, 95% CI = 0.18 - 0.66), confusion (OR = 2.15, 95% CI = 1.57 - 2.94), aphasia (OR = 1.18, 95% CI = 0.47 - 2.95), motor deficit (OR = 3.07, 95% CI = 2.66 - 3.55), papilledema (OR = 0.36, 95% CI = 0.22 - 0.58), sensory disturbance (OR = 1.73, 95% CI = 0.45 - 6.67), nausea/vomiting (OR = 0.77, 95% CI = 0.58 - 1.03). This result demonstrates strong association of frontal lobe, parietal lobe, cortical vein, hemorrhagic ischemia, confusion, and motor deficit with seizure occurrence in CVST.
 Click to view | Table 1. Odds Ratio (OR) Calculated by the Random-Effects Model for Seizure Occurrence Between Cerebral Venous Thrombosis Patients With and Without Each Risk Factor Studied |
Involvement of frontal or parietal lobe, cortical vein, presence of hemorrhagic venous ischemia, motor deficit, or confusion were found to be significantly associated with acute symptomatic seizure in the acute phase of CVST. Meta-regression, including age, sex, study type, country, time frame (year the study began; ended), and study duration was performed to identify the potential source of heterogeneity. Only the starting year of the study significantly contributed to heterogeneity (R2 = 32.4%, P < 0.0001).
We constructed the following formula using the significant risk factors as follows: for radiographic finding (R), frontal lobe, parietal lobe, cortical vein, and hemorrhagic venous ischemia; for clinical presentation (C), motor deficit and confusion (Table 2). β-coefficient values were 1.58 for frontal lobe, 0.92 for parietal lobe, 1.15 for cortical vein, 1.35 for hemorrhagic venous ischemia; 0.77 for motor deficit, and 1.12 for confusion. A point value of 0.5 was assigned to risk factors with β-coefficient below 1. Risk factors with β-coefficient between 1 and 1.3 were given a point value of 1. Three points were given to risk factors with β-coefficient greater than 1.3. For R in radiographic finding and C in clinical presentation, we named this novel score system as Radiographic and Clinical Assessment (RC) scoring system. This score system was developed based on meta-analyses of 14 studies with total 4,414 patients.
 Click to view | Table 2. A Novel Radiographic and Clinical Assessment (RC) Scoring System |
Discriminative ability of this novel prediction score was assessed using the receiver operating characteristic curve. This prediction score yielded a calculated area under the curve (AUC) of 0.89, with probabilities for seizure ranging from 40% with a score of 0 to 92% for score of 6 (Table 3). AUC of 0.89 demonstrates an excellent discriminative ability of this prediction model. Starting point of this curve was set at 0 point with 40% probably as it is the reported probability of having a seizure in patients with CVST in general [14]. Patients with RC score 2 or greater should be considered for AED prophylaxis as their seizure probability is greater than 50%.
 Click to view | Table 3. Score Interpretation for the Radiographic and Clinical assessment (RC) Scoring System |
| Discussion | ▴Top |
Herein, both motor deficits and cortical vein thrombosis were strongly associated with seizure risk. Both factors could be acting as a surrogate for cortical damage, which is well known to be of a higher risk for seizures. However, even if these are a surrogate marker, we have shown how there is a significant link between motor deficit and seizure, making it a reasonable inclusion in this score. Furthermore, the cortical damage may also explain the association of those patients with presenting with focal deficit having higher association of seizure. This same concept can be applied to the possible rational behind the strong association between seizure in acute CVST and frontal lobe involvement that may be due to frontal lobes being the venous territory corresponding to the cortical vein and superficial sagittal sinus [34]. CVST in superficial sagittal sinus/cortical veins will obstruct the flow, leading to focal edema and venous ischemia.
Although has been a paradigm shift throughout the history of neurologic practice away from obligatory seizure prophylaxis this has always been a risk verse benefit assessment made by the clinician. Many anti-seizure medications come with a notable degree of risk due to or interactions with medications or side effects, including liver injury, hypotension, and cardiac arrhythmia. The benefit, seizure reduction, would only be seen in those who would have had a seizure without the medications, in other words those at high risk. By stratifying the seizure risk of patients, through such scoring tools as this, we can limit the risks from these medications by limiting through use to those most likely to benefit. In view of these problems associated with AEDs, judicious use of AEDs based on the sound evidence is critical. For this reason, the available evidence was critically evaluated in this systematic review with meta-analysis for primary prophylactic use of AEDs in the acute phase of CVST.
In previous meta-analysis published by Li et al in 2019, six observational studies including total 1,244 patients were analyzed. This study identified a positive association of acute seizure in CVST with frontal or parietal lobe, cortical vein, hemorrhagic ischemia, altered mental status and motor deficit [35]. This research finding is consistent with the outcome of our study.
The main highlight of our index is the large sample of patients, consisting of 4,414 individuals, from 14 studies. This analysis combined the observational studies to amplify the statistical precision and power. Based on the significant risk factors identified in this work, we constructed the RC scoring system to predict the seizure risk in CVST. This score system is a new contribution to what is known of the seizure risk in the acute phase of CVST. It is our hope RC score system could be used to help guide the judicious use of anti-epileptic therapy in this patient population.
While this score was derived from extensive review of the literature, the largest study we are aware to date, it has yet to be validated in clinical practice, demonstrating that the seizure frequency falls within the expected range and the concordance between both the radiographic finding and clinical presentation to the seizure risk. Further studies could investigate all-cause mortality between those who are treated with prophylactic seizure medications and those who are not, when looking at patient from each score value. It could be used for early seizure prophylaxis management. Early prediction is important for controlling seizures and preventing secondary brain damage. Subsequently, we developed the nomogram for predicting this complication for an individual patient in real practice.
Using Web of Science, and Pro-Quest databases, this limited search likely has limited generalization of our index. This study is based on pooled analysis of aggregate patient data. For this reason, patient characteristics at individual level was not available to our study. Important differences across studies, such as the percentage of pregnancy-related CVST, were not taken into consideration for the summary effect.
Given a rarity of CVST, validation study for the RC score system would be a challenge. Collaboration of multiple stroke centers across the country would be required for the validation study in clinical practice. Further studies could investigate all-cause mortality between those are treated with prophylactic seizure medications and those who are not, when looking at patient from each score value.
In severe traumatic brain injury (TBI), the probability of acute seizure within 7 days of injury is reported to be about 12%. AED prophylaxis in this setting has shown to decrease the occurrence of acute seizure by 75%. Based on this evidence, current guideline from The Brain Trauma Foundation recommends primary AED prophylaxis in patients with severe TBI for 7 days after injury [36]. Looking along the same line, the efficacy of AED prophylaxis in CVST will need to be evaluated. This information would be useful in risk-benefit analysis for use of RC score in routine clinical practice.
Conclusions
The RC score system, combining information about vascular and lobar localization, type of ischemia, and clinical presentation, may be a useful predictor for seizure development in CVST patients.
| Supplementary Material | ▴Top |
Suppl 1. Search term used in MEDLINE, EBSCO, Web of Science, and Pro-Quest Databases.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors have no conflict of interest to disclose.
Informed Consent
Not applicable.
Author Contributions
Stella Pak: manuscript writing, revision, systematic review, and meta-analysis. Sahil Sardana: revision, editing and review. Rudy Estess: manuscript writing, revision, systematic review, and meta-analysis. Nihita Manem: systematic review and revision. Tamer Abdelhak: editing, reviewing, and research design.
Data Availability
The data supporting the findings are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Proposal for revised classification of epilepsies and epileptic syndromes. Commission on classification and terminology of the international league against epilepsy. Epilepsia. 1989;30(4):389-399.
doi pubmed - Hawkes MA, Hocker SE. Systemic complications following status epilepticus. Curr Neurol Neurosci Rep. 2018;18(2):7.
doi pubmed - Scoppettuolo P, Gaspard N, Depondt C, Legros B, Ligot N, Naeije G. Epileptic activity in neurological deterioration after ischemic stroke, a continuous EEG study. Clin Neurophysiol. 2019;130(12):2282-2286.
doi pubmed - Witsch J, Frey HP, Schmidt JM, Velazquez A, Falo CM, Reznik M, Roh D, et al. Electroencephalographic periodic discharges and frequency-dependent brain tissue hypoxia in acute brain injury. JAMA Neurol. 2017;74(3):301-309.
doi pubmed - Wang JZ, Vyas MV, Saposnik G, Burneo JG. Incidence and management of seizures after ischemic stroke: Systematic review and meta-analysis. Neurology. 2017;89(12):1220-1228.
doi pubmed - Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110.
doi pubmed - Holtkamp M, Beghi E, Benninger F, Kalviainen R, Rocamora R, Christensen H, European Stroke Organisation. European Stroke Organisation guidelines for the management of post-stroke seizures and epilepsy. Eur Stroke J. 2017;2(2):103-115.
doi pubmed - Katsiki N, Mikhailidis DP, Nair DR. The effects of antiepileptic drugs on vascular risk factors: a narrative review. Seizure. 2014;23(9):677-684.
doi pubmed - Goldstein LB. Common drugs may influence motor recovery after stroke. The sygen in acute stroke study investigators. Neurology. 1995;45(5):865-871.
doi pubmed - Troisi E, Paolucci S, Silvestrini M, Matteis M, Vernieri F, Grasso MG, Caltagirone C. Prognostic factors in stroke rehabilitation: the possible role of pharmacological treatment. Acta Neurol Scand. 2002;105(2):100-106.
doi pubmed - Scales DC, Fischer HD, Li P, Bierman AS, Fernandes O, Mamdani M, Rochon P, et al. Unintentional continuation of medications intended for acute illness after hospital discharge: a population-based cohort study. J Gen Intern Med. 2016;31(2):196-202.
doi pubmed - Coutinho JM, Zuurbier SM, Aramideh M, Stam J. The incidence of cerebral venous thrombosis: a cross-sectional study. Stroke. 2012;43(12):3375-3377.
doi pubmed - Koton S, Schneider AL, Rosamond WD, Shahar E, Sang Y, Gottesman RF, Coresh J. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA. 2014;312(3):259-268.
doi pubmed - Ferro JM, Canhao P, Stam J, Bousser MG, Barinagarrementeria F, Investigators I. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35(3):664-670.
doi pubmed - Patel ND, Desai N, MR SS, Patel SD, Tunguturi A, Mahuwala ZK. Burden of acute symptomatic seizures in cerebral venous sinus thrombosis: A nationwide United States analysis. Clin Neurol Neurosurg. 2021;209:106943.
doi pubmed - Filippidis A, Kapsalaki E, Patramani G, Fountas KN. Cerebral venous sinus thrombosis: review of the demographics, pathophysiology, current diagnosis, and treatment. Neurosurg Focus. 2009;27(5):E3.
doi pubmed - Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6(2):162-170.
doi - Saposnik G, Barinagarrementeria F, Brown RD, Jr., Bushnell CD, Cucchiara B, Cushman M, deVeber G, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158-1192.
doi pubmed - Price M, Gunther A, Kwan JS. Antiepileptic drugs for the primary and secondary prevention of seizures after intracranial venous thrombosis. Cochrane Database Syst Rev. 2016;4:CD005501.
doi pubmed - Ferro JM, Correia M, Rosas MJ, Pinto AN, Neves G, Cerebral Venous Thrombosis Portuguese Collaborative Study G. Seizures in cerebral vein and dural sinus thrombosis. Cerebrovasc Dis. 2003;15(1-2):78-83.
doi pubmed - Ferro JM, Canhao P, Bousser MG, Stam J, Barinagarrementeria F, Investigators I. Early seizures in cerebral vein and dural sinus thrombosis: risk factors and role of antiepileptics. Stroke. 2008;39(4):1152-1158.
doi pubmed - Kalita J, Chandra S, Misra UK. Significance of seizure in cerebral venous sinus thrombosis. Seizure. 2012;21(8):639-642.
doi pubmed - Davoudi V, Keyhanian K, Saadatnia M. Risk factors for remote seizure development in patients with cerebral vein and dural sinus thrombosis. Seizure. 2014;23(2):135-139.
doi pubmed - Ding H, Xie Y, Li L, Chu H, Tang Y, Dong Q, Cui M. Clinical features of seizures after cerebral venous sinus thrombosis and its effect on outcome among Chinese Han population. Stroke Vasc Neurol. 2017;2(4):184-188.
doi pubmed - Singh RK, Bhoi SK, Kalita J, Misra UK, Gupta D. A comparative study of seizures in arterial and venous stroke. International Journal of Epilepsy. 2017;4(1):6-11.
doi - Sha DJ, Qian J, Gu SS, Wang LN, Wang F, Xu Y. Cerebral venous sinus thrombosis complicated by seizures: a retrospective analysis of 69 cases. J Thromb Thrombolysis. 2018;45(1):186-191.
doi pubmed - Kalita J, Singh VK, Jain N, Misra UK, Kumar S. Cerebral venous sinus thrombosis score and its correlation with clinical and MRI findings. J Stroke Cerebrovasc Dis. 2019;28(11):104324.
doi pubmed - Gazioglu S, Yildirim A, Kokturk EG, Seker D, Altunayoglu Cakmak V, Velioglu SK. Acute seizures in cerebral venous sinus thrombosis: risk factors and prognosis. Neurologist. 2020;25(5):126-130.
doi pubmed - Goyal G, Singh R. Predictors of presenting seizures in acute cerebral vein and dural sinus thrombosis. J Epilepsy Res. 2020;10(2):74-78.
doi pubmed - Lindgren E, Silvis SM, Hiltunen S, Heldner MR, Serrano F, de Scisco M, Zelano J, et al. Acute symptomatic seizures in cerebral venous thrombosis. Neurology. 2020;95(12):e1706-e1715.
doi pubmed - Uluduz D, Midi I, Duman T, Yayla V, Karahan AY, Afsar N, Goksu EO, et al. Epileptic seizures in cerebral venous sinus thrombosis: Subgroup analysis of VENOST study. Seizure. 2020;78:113-117.
doi pubmed - Fang Y, Song G, Lin J, Ye X, Huang S. Predicting the occurrence of early seizures after cerebral venous thrombosis using a comprehensive nomogram. Epilepsy Res. 2021;178:106820.
doi pubmed - Amornpojnimman T, Mitarnun W, Korathanakhun P. Predictors of seizures in patients with cerebral venous thrombosis in the Thai population: A retrospective study. Seizure. 2022;96:1-5.
doi pubmed - Kilic T, Akakin A. Anatomy of cerebral veins and sinuses. Front Neurol Neurosci. 2008;23:4-15.
doi pubmed - Li H, Cui L, Chen Z, Chen Y. Risk factors for early-onset seizures in patients with cerebral venous sinus thrombosis: A meta-analysis of observational studies. Seizure. 2019;72:33-39.
doi pubmed - Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80(1):6-15.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.
