| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website http://www.neurores.org |
Original Article
Volume 9, Number 4-5, October 2019, pages 65-71
The Association of Optical Coherence Tomography Results With Neuroimaging Signs and Some Clinical Parameters in Idiopathic Intracranial Hypertension
Halil Ondera, c, Erol Erkanb
aNeurology Clinic, Yozgat City Hospital, Yozgat, Turkey
bOphthalmology Clinic, Yozgat City Hospital, Yozgat, Turkey
cCorresponding Author: Halil Onder, Neurology Clinic, Yozgat City Hospital, Yozgat, Turkey
Manuscript submitted August 12, 2019, accepted September 27, 2019
Short title: Optical Coherence Tomography in IIH
doi: https://doi.org/10.14740/jnr550
| Abstract | ▴Top |
Background: Recently, optical coherence tomography (OCT) has enhanced our understanding of visual disturbances in idiopathic intracranial hypertension (IIH). Its importance in the evaluation process of IIH has been established; however, there are many unknown aspects regarding the relationship of OCT measurements with several clinical features of IIH. Herein, we aimed to investigate the associations of OCT measurements with neuroimaging findings and some clinical parameters in our cohort with IIH.
Methods: Patients over 18 years of age presenting to the neurology and neuro-ophthalmology outpatient clinics, between 2017 and 2019, who were diagnosed with IIH were included in the study. Cranial magnetic resonance imaging (MRI) recordings were retrospectively evaluated for the presence of neuroimaging signs of intracranial hypertension. Peripapillary retinal nerve fiber layer (RNFL) measurements and other clinical parameters were retrospectively evaluated from the hospital recoding system. SPSS Statistics (version 20) were used for statistical analyses.
Results: We have included 18 patients of IIH with a mean age of 38.6 years (range: 19 - 69 years) and female/male ratio was 17:1. Mean body mass index (BMI) of the patients was 30.5 ± 5.7 and mean lumbar puncture (LP) opening pressure was 313.8 ± 66.6 mm H2O. Correlation analyses between LP opening pressure and average RNFL thickness of the right eye revealed a significant positive correlation. The results of the other correlation analyses were unremarkable.
Conclusions: We have found a significant correlation between LP opening pressure and RNFL thickness of the right eye. No association between RNFL measurement and MRI signs of intracranial hypertension was found. Investigating the possible associations between RNFL measurements and the clinical and neuroimaging signs in future studies may provide crucial contributions regarding the unknown aspects of IIH pathophysiology.
Keywords: Peripapillary RNFL; Optical coherence tomography; Idiopathic intracranial hypertension; Neuroimaging; Pathophysiology
| Introduction | ▴Top |
Idiopathic intracranial hypertension (IIH) is characterized by diffuse, chronic headache and visual disturbances whose diagnostic criteria include papilledema, normal cerebral magnetic resonance imaging (MRI) and cerebrospinal fluid (CSF) composition, normal neurological examination except for the sixth nerve abnormalities and increased CSF pressure (> 25 cm H2O) measured by lumbar puncture (LP) [1]. Imaging findings such as an empty sella, optic nerve head (ONH) protrusion, posterior scleral flattening or increased perioptic CSF may support the diagnosis [2]. Optical coherence tomography (OCT) has been identified as a crucial method to accurately monitor the ONH volume and neuroaxonal retinal damage [3]. Remarkably, the utility of OCT technique in the clinical evaluation processes of IIH has been shown in multiple studies, previously [4-8]. The results of these studies have shown increased ONH volume in IIH patients [4], decreasing ONH volume on treatment for IIH [5]. In conclusion, it has been suggested that retinal nerve fiber layer (RNFL) thickness (a measurement of retinal fiber thickness on OCT) can be a more reliable criterion for quantitating the papilledema compared with ophthalmoscopy alone, which is subjective and may reflect significant interobserver variability [9]. However, there are still debates about the function of ONH volume measurements as well as the pathophysiology which they are reflecting. We think that the investigation of OCT results in these patients and comparing them with clinical features may provide substantial perspectives regarding the unknown aspect of the IIH pathophysiology. Therefore, herein, we aimed to investigate the associations of OCT measurements of peripapillary RNFL thickness with neuroimaging findings and some clinical parameters in our cohort with IIH.
| Materials and Methods | ▴Top |
Patients over 18 years of age presenting to the neurology and neuro-ophthalmology outpatient clinics, between 2017 and 2019 with bilateral papilledema and no localized neurologic findings other than abducens palsy, if a diagnosis of intracranial mass lesion and hydrocephalus was excluded by imaging and an initial measured CSF pressure of ≥ 250 cm H2O, were included. Modified Dandy criteria were used for the diagnosis of IIH. Written informed consents were obtained from all patients and this study was approved by the Institutional Review Board. The study was retrospective in design. Clinical, neuroimaging and OCT measurements of these patients were retrospectively evaluated. Patients who were not investigated with OCT at admission (prior to LP) were excluded from the study.
The clinical manifestations of the patients, visual examinations and follow-up data of them were evaluated. Body mass index (BMI) was calculated for each patient. Neuro-ophthalmological examination, visual field testings (VFT) with a Humphrey Field Analyzer at the ophthalmology unit and OCT tests were performed. Cranial MRI and magnetic resonance venography (MRV) had been performed in a single session in all the patients using a 1.5 Tesla Magnetom Amira MRI scanner (5-mm slice thickness).
The images were assessed by a neurologist (HO) in the supervision of a radiology specialist (TK) who is particularly interested in neuroimaging. Conventional cranial MRI studies were evaluated in terms of ONH protrusion, posterior scleral flattening, increased perioptic CSF, optic nerve tortuosity, partial empty sella, tonsillar herniation, enlargement of Meckel’s cave, and meningoceles. However, it was realized that the quality of MRV images was insufficient to be used for interpretations; and therefore, they were excluded from analyses. Unfortunately, transverse sinus stenosis (TSS) could not be evaluated due to the suboptimal images of MRV. Partial empty sella was defined as the presence of CSF in > 50% the pituitary fossa [10]. Posterior flattening of the globe was diagnosed when the normal convexity at the origin of the optic nerve was flattened in the orbital MRI [11]. Optic nerve protrusion was defined as the loss of its convexity at the point of entry into the globe and attainment of a concave form, as shown by MRI [12]. Vertical tortuosity of the optic nerve is defined as the presence of an “S” shaped optic nerve in sagittal cranial MR images. Increased perioptic CSF is diagnosed in the presence of a calculated CSF distance of > 2 mm around the optic nerve in coronal cross-sections [10]. Inferior tonsillar displacement is defined as a > 5 mm downward displacement of cerebellar tonsils as compared to normal anatomical location. Enlargement of Meckel’s cave was evaluated as prominent or increased fluid signal expanding Meckel’s cave but not distorting the contours [13]. LP had been performed in all of the patients after radiological examination, and CSF opening pressure, cell content, and biochemistry were evaluated.
Both eyes of patients who met the study criteria underwent a complete ophthalmic evaluation including Snellen visual acuity (VA), biomicroscopy of the anterior and posterior segments, automated perimetry (Swedish Interactive Threshold Algorithm standard 24-2 strategy, Humphrey Visual Field Analyzer; Carl Zeiss Meditec, Dublin, CA), and OCT imaging. A 90-D lens biomicroscopic assessment of the degree of papilledema was based on the scheme proposed by Frisen. No patients had signs of atrophic papilledema. OCT scanning was performed (Cirrus 5000 HD OCT; Carl Zeiss Meditec) after pharmacologic mydriasis with 1% tropicamide. Image acquisition was performed with the Fast RNFL Thickness (3.46) strategy. Satisfactory image quality was defined as good centration on the optic disc and signal strength of 7 or greater. RNFL thicknesses were obtained with the built-in OCT software RNFL Thickness Average Analysis protocol. A color-coded graph displays the RNFL measurements and compares them with the age-matched data of a normative database.
Statistical analyses were performed to compare the neuroimaging findings in IIH and cerebral venous thrombosis (CVT) groups in order to detect any difference that can be meaningful in the diagnosis. A value of total MRI score was formed by adding the number of existing abnormal MRI parameters among all the eight specified criteria. This score was used while evaluating the association between MRI findings and clinical parameters, LP opening pressure in the correlation analyses and comparative analyses. In addition, the diagnostic sensitivities of all the MRI parameters were evaluated separately. Besides, the diagnostic sensitivities of a combination of any two findings in patients with IIH were also calculated. Continuous variables were assessed using mean ± standard deviation and median (minimum - maximum), while categorical variables were summarized with percentages. The between-group comparisons for continuous variables were performed with independent t-test, while Chi-square and Fisher exact tests were used for the categorical data. The correlations between two continuous variables were evaluated with Pearson correlation. Univariate linear regression analyses were also performed to exclude the effect of BMI while evaluating the association between MRI scores and LP opening pressures. Levene’s test was used to compare the means of some clinical parameters between distinct IIH groups those classified according to a cut-off value of LP opening pressure (300 mm H2O). All statistical analyses were performed with SPSS Statistics for Windows (version 20, IBM Corp., Armonk, NY, USA). A P value < 0.05 was considered statistically significant.
| Results | ▴Top |
We have included 18 patients of IIH with a mean age of 38.6 years (range: 19 - 69 years) and female/male ratio was 17:1. Mean BMI of the patients was 30.5 ± 5.7, which was higher than the obesity threshold. Mean LP opening pressure was 313.8 ± 66.6 mm H2O, and total MRI score, evaluating the sum of positivity of the eight findings of intracranial hypertension was 2.5 ± 1.2. OCT measurements revealed that the average RNFL thickness was 113.8 ± 52.9 µm in the right eye, whereas it was 111.3 ± 47.2 µm in the left eye (Table 1).
 Click to view | Table 1. Clinical Features of the Patients |
Correlation analyses between LP opening pressure and average RNFL thickness of the right eye revealed a significant positive correlation (r: 0.470; P value: 0.049) (Fig. 1). The value of the correlation coefficient between LP opening pressure and the RNFL thickness of the left eye was 0.440 (P = 0.067), whereas it was 0.427 (P = 0.077) with the mean RNFL thickness. The results of the other correlation analyses investigating the association with other clinical and neuroimaging features were non-significant (Table 2).
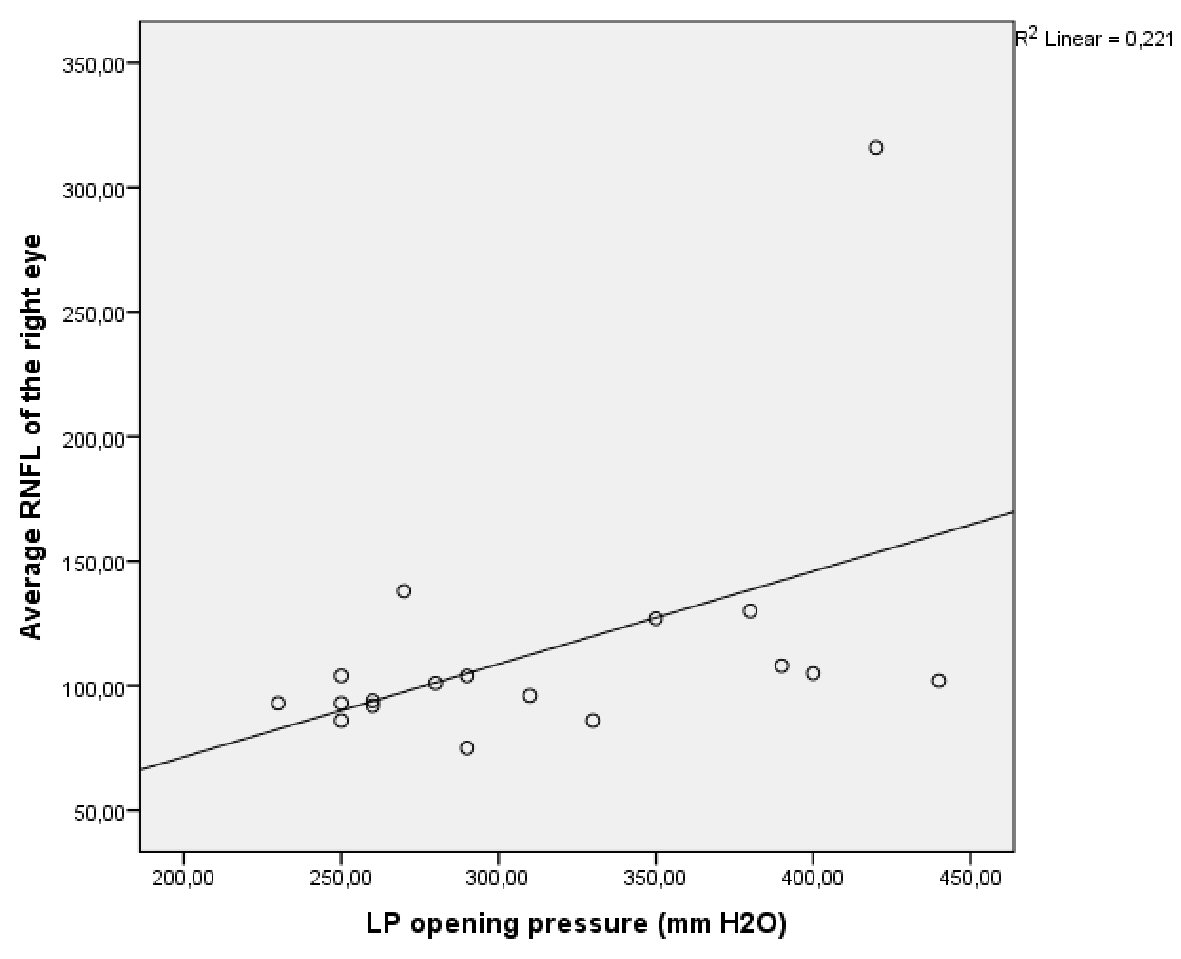 Click for large image | Figure 1. Graphic analysis demonstrating the correlation between LP opening pressure and average RNFL thickness of the right eye. LP: lumbar puncture; RNFL: retinal nerve fiber layer. |
 Click to view | Table 2. Correlation Analyses Between OCT and MRI Measurements and Some Clinical Features |
We have also made comparative analyses after categorizing the patients according to LP opening pressure value. The results of the initial analyses according to the cut-off value of 300 mm H2O revealed that BMI values were higher in the patient group with higher LP opening pressure values (P = 0.046). The results of the other comparisons were unremarkable (Table 3). The analyses were also repeated after categorizing the patients according to a cut-off value of 400 mm H2O. The results of the latter analyses revealed that mean RNFL measurements were significantly higher in the patient group with LP opening pressure higher than 400 mm H2O (Table 4).
 Click to view | Table 3. T-Test Comparing the Clinical, MRI and OCT Measurements Between Patient Groups Categorized According to Cut-Off LP Opening Pressure of 300 mm H2O (Levene’ s Test) |
 Click to view | Table 4. T-Test Comparing the Clinical, MRI and OCT Measurements Between Patient Groups Categorized According to Cut-Off LP Opening Pressure of 400 mm H2O (Levene’s Test) |
Among the neuroimaging parameters, the most common two findings were increased perioptic CSF (83%) and optic nerve tortuosity (50%), respectively (samples of some neuroimaging signs from our cohort) (Table 5 and Fig. 2).
 Click to view | Table 5. Frequencies of the MRI Signs of Intracranial Hypertension in Our Patient Group |
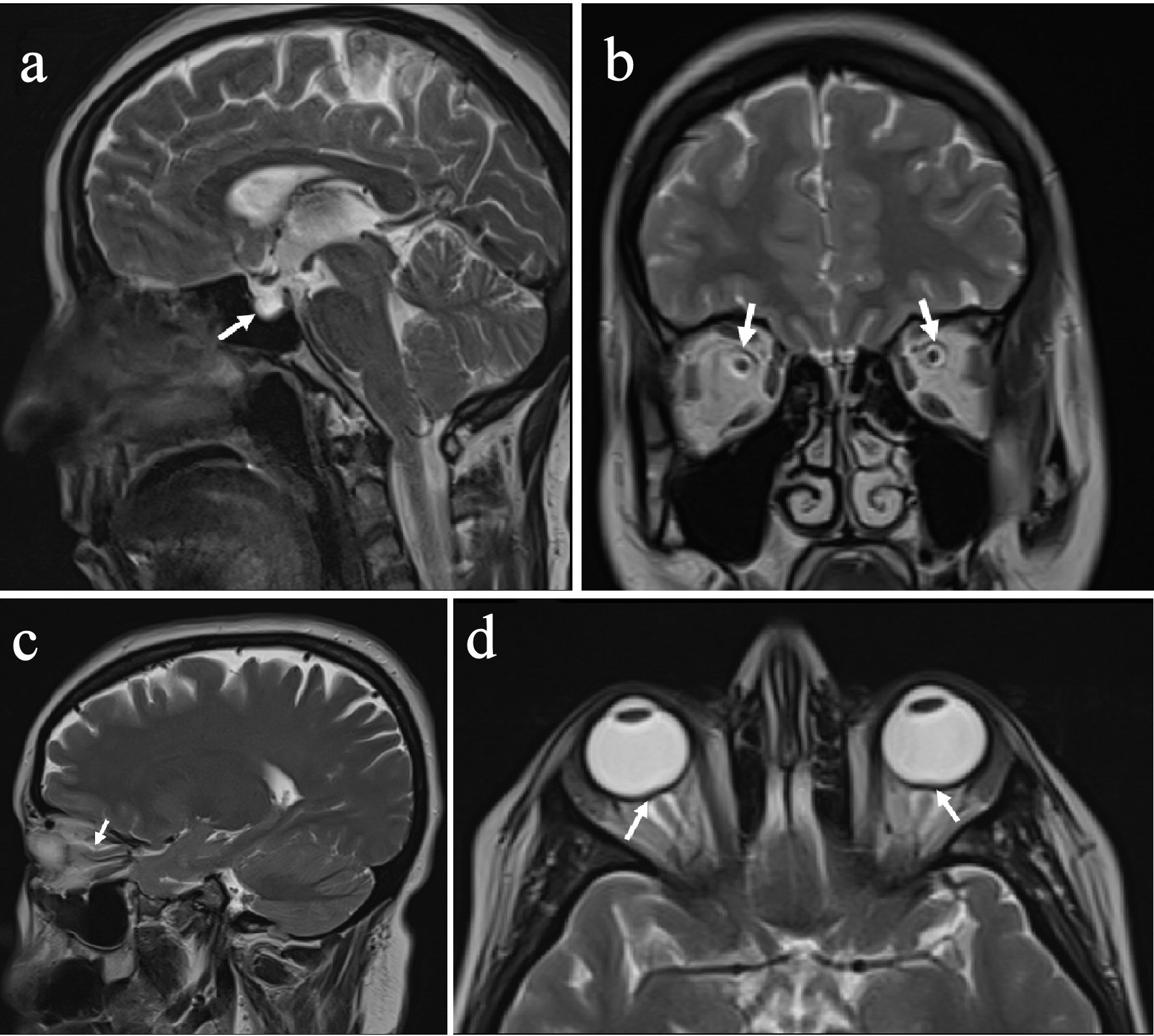 Click for large image | Figure 2. Images from the patients with IIH. (a) Empty sella. (b) Increased perioptic CSF. (c) Optic nerve tortuosity. (d) Optic nerve head protrusion (arrows). IIH: idiopathic intracranial hypertension; CSF: cerebrospinal fluid. |
| Discussion | ▴Top |
In this study, we have found that RNFL measurements were significantly higher in the patients with LP opening pressure more than 400 mm H2O. However, no a difference was found when the cut-off value was determined as 300 mm H2O. Correlation analyses revealed a significant correlation between LP opening pressure and RNFL thickness of the right eye and a trend towards significance in the left eye measurements (r = 0.440, P = 0.067). In literature, some reports showed positive correlations between the CSF opening pressure and peripapillary RNFL measurements [7, 14]. However, there are also other authors reporting non-association between the CSF opening pressure and RNFL thickness [6, 8], as in our report. Previous reports regarding the utility of OCT in the clinical evaluation processes of IIH have shown positive correlations between papilledema grade and RNFL measurements multiple times, confidingly [6, 7, 15]. However, it is known that LP opening pressure, which is a vital criterion for the diagnosis of IIH, has no a validated utility in prognostication of the patients as well as predicting the visual disturbances in the acute period or long term. Besides, the association between papilledema grade and LP opening pressure has not even been reliably demonstrated in the literature. Therefore, we think that the results of the previous reports supporting the association between LP opening pressure and OCT measurements should be interpreted cautiously to avoid misleading conclusions. Actually, there may be several additional factors influencing the clinical outcome of papilledema (other than LP opening pressure, a criterion reflecting the increased intracranial pressure) and its causal relationship with the degree of intracranial hypertension, such as duration of the disease, intraocular pressure, possible differing intracranial pressure dynamics individually as well as the differing dura histology and physiology which plays a critical role in transmission of the increased pressure [16]. Taken together, while interpreting the results of the studies investigating the association between LP opening pressure and RNFL thickness, we should keep in mind that many of these confounding factors would be involved in this association (whose exclusion would not be possible). In accordance with this thought, we have found a significant difference in RNFL measurements when we grouped the patients according to the LP opening pressure of 400 mm H2O; however, this difference was not present between the patient groups categorized according to the cut-off value of 300 mm H2O. We think that in substantial values of intracranial pressure increments, the effects of these potential confounding factors might be insufficient to affect the RNFL values, which was probably not the case in the latter categorization (according to 300 mm H2O).
Another critical conclusion was that we have not found a correlation between total MRI score of intracranial hypertension and LP opening pressure measurements. In a unique study by Tuncel et al, also no correlation was found similar to our report [17]. In another study by Saindane et al, an advanced MR imaging technique highly sensitive to brain motion was used to show the correlations between pontine displacement and LP opening pressure which yielded positive results [18]. However, in this study, the conventional neuroimaging findings of intracranial hypertension (investigated in our study) were not evaluated. Ergo, we think that the results of our study investigating the relationship between LP opening pressure and neuroimaging signs, which are strictly rare in literature, are crucial in this regard. On the other hand, we also evaluated the frequency of these signs one by one in our patient group, in conclusion of which we have found significantly high rates of the presence of these findings. The sensitivity of these MRI signs in patients with IIH varies widely between studies, with reported rates of between 6% and 72% [2, 19]. Remarkably, we have determined that the increased perioptic CSF was present in 83% of the patients and optic nerve tortuosity was present in 50% of them, which were significantly high according to the previously reported rates. Nevertheless, our patient group was not wide enough to make ambitious conclusions on this issue. Besides, interpreter of the MRIs was not blinded to the diagnosis, which might have led a bias effect in the results.
Another crucial issue in this regard may be the relationship between BMI and IIH [20-22]. The association between increased BMI and increased risk of IIH has been well established in the literature. However, the causal relationship between these two entities, and its BMI relationship to visual outcomes in IIH are issues which remain to be elucidated. The mean BMI was 30.5 ± 5.7 in our group which was higher than the limit for obesity. However, it is remarkable to state that we have not found any association between BMI and LP opening pressure, the total MRI score or RNFL measurements. On the other hand, BMI was significantly higher in the patient group with LP opening pressure of ≥ 300 mm H2O in comparison to the remaining patient group. Of note, when the analyses were repeated after categorizing according to the cut-off value of 400 mm H2O, the significance did not persist. We interpret these results as that the high mean of BMI in our cohort was in accordance with the literature evidence emphasizing obesity as a risk factor of IIH. However, negative correlation results and non-significance of the differences of BMI in the subgroup with strictly high LP opening pressure also support that obesity does not play an active role during the course of the disease. Future large-scale studies are surely warranted to clarify these discussions.
In conclusion, we have found a significant correlation between LP opening pressure and RNFL thickness of the right eye. However, measurements of RNFL thickness were significantly higher in the patient group with LP opening pressure higher than 400 mm H2O. No association between RNFL measurement and MRI signs of intracranial hypertension was found. Future large-scale studies are warranted to clarify our conclusions. Investigating the possible associations between RNFL measurements and the clinical and neuroimaging signs in these studies may provide crucial contributions regarding the unknown aspects of IIH pathophysiology.
Acknowledgments
We thank TK for all the support during the evaluation process of MRIs.
Conflict of Interest
None to declare.
Financial Disclosure
None to declare.
Informed Consent
Informed consents have been obtained from the patients.
Author Contributions
HO contributed substantially to clinical evaluation, conception and design of the report, writing, critical review and submission of the manuscript. EE contributed substantially to clinical evaluation and critical review of the manuscript.
| References | ▴Top |
- Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59(10):1492-1495.
doi pubmed - Kwee RM, Kwee TC. Systematic review and meta-analysis of MRI signs for diagnosis of idiopathic intracranial hypertension. Eur J Radiol. 2019;116:106-115.
doi pubmed - Kaufhold F, Kadas EM, Schmidt C, Kunte H, Hoffmann J, Zimmermann H, Oberwahrenbrock T, et al. Optic nerve head quantification in idiopathic intracranial hypertension by spectral domain OCT. PLoS One. 2012;7(5):e36965.
doi pubmed - Batra R, Sinclair A. Idiopathic intracranial hypertension; research progress and emerging themes. J Neurol. 2014;261(3):451-460.
doi pubmed - Albrecht P, Blasberg C, Ringelstein M, Muller AK, Finis D, Guthoff R, Kadas EM, et al. Optical coherence tomography for the diagnosis and monitoring of idiopathic intracranial hypertension. J Neurol. 2017;264(7):1370-1380.
doi pubmed - D.H.A.R. Labib DM. Diagnostic value of optical coherence tomography in patients with idiopathic intracranial hypertension. The Egyptian Journal of Neurology, Psychiatry and Neurosurgery. 2015;52:249-253.
doi - Group OCTS-SCfNIIHS, Auinger P, Durbin M, Feldon S, Garvin M, Kardon R, Keltner J, et al. Baseline OCT measurements in the idiopathic intracranial hypertension treatment trial, part I: quality control, comparisons, and variability. Invest Ophthalmol Vis Sci. 2014;55(12):8180-8188.
doi pubmed - Rebolleda G, Munoz-Negrete FJ. Follow-up of mild papilledema in idiopathic intracranial hypertension with optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50(11):5197-5200.
doi pubmed - Huang-Link YM, Al-Hawasi A, Oberwahrenbrock T, Jin YP. OCT measurements of optic nerve head changes in idiopathic intracranial hypertension. Clin Neurol Neurosurg. 2015;130(122-127.
doi pubmed - Brodsky MC, Vaphiades M. Magnetic resonance imaging in pseudotumor cerebri. Ophthalmology. 1998;105(9):1686-1693.
doi - Brodsky MC. Flattening of the posterior sclera: hypotony or elevated intracranial pressure? Am J Ophthalmol. 2004;138(3):511; author reply 511-512.
doi pubmed - Jinkins JR, Athale S, Xiong L, Yuh WT, Rothman MI, Nguyen PT. MR of optic papilla protrusion in patients with high intracranial pressure. AJNR Am J Neuroradiol. 1996;17(4):665-668.
- Bialer OY, Rueda MP, Bruce BB, Newman NJ, Biousse V, Saindane AM. Meningoceles in idiopathic intracranial hypertension. AJR Am J Roentgenol. 2014;202(3):608-613.
doi pubmed - Heckmann JG, Weber M, Junemann AG, Neundorfer B, Mardin CY. Laser scanning tomography of the optic nerve vs CSF opening pressure in idiopathic intracranial hypertension. Neurology. 2004;62(7):1221-1223.
doi pubmed - Vartin CV, Nguyen AM, Balmitgere T, Bernard M, Tilikete C, Vighetto A. Detection of mild papilloedema using spectral domain optical coherence tomography. Br J Ophthalmol. 2012;96(3):375-379.
doi pubmed - Chisholm JT, Sudhakar P, Alhajeri AN, Smith JH. Intracranial elastance is increased in idiopathic intracranial hypertension. Eur J Neurol. 2017;24(12):1457-1463.
doi pubmed - Tuncel SA, Yilmaz E, Cagli B, Tekatas A, Celik Y, Unlu ME. Lumbar opening pressure and radiologic scoring in idiopathic intracranial hypertension: is there any correlation? Pol J Radiol. 2017;82(701-705.
doi pubmed - Saindane AM, Qiu D, Oshinski JN, Newman NJ, Biousse V, Bruce BB, Holbrook JF, et al. Noninvasive assessment of intracranial pressure status in idiopathic intracranial hypertension using Displacement Encoding with Stimulated Echoes (DENSE) MRI: a prospective patient study with contemporaneous CSF pressure correlation. AJNR Am J Neuroradiol. 2018;39(2):311-316.
doi pubmed - Bidot S, Saindane AM, Peragallo JH, Bruce BB, Newman NJ, Biousse V. Brain imaging in idiopathic intracranial hypertension. J Neuroophthalmol. 2015;35(4):400-411.
doi pubmed - Sugerman HJ, Felton WL, 3rd, Salvant JB, Jr., Sismanis A, Kellum JM. Effects of surgically induced weight loss on idiopathic intracranial hypertension in morbid obesity. Neurology. 1995;45(9):1655-1659.
doi pubmed - Hannerz J, Ericson K. The relationship between idiopathic intracranial hypertension and obesity. Headache. 2009;49(2):178-184.
doi pubmed - Hannerz J, Greitz D, Ericson K. Is there a relationship between obesity and intracranial hypertension? Int J Obes Relat Metab Disord. 1995;19(4):240-244.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.
