| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website http://www.neurores.org |
Case Report
Volume 10, Number 3, June 2020, pages 91-94
A Case of Unsteady Gait: An Amyotrophic Lateral Sclerosis Mimic Causing Reversible Weakness
Paola Martineza, b, Jonathan Donnellya, Humberto Leal-Baileya, Kyna Schreibera, Alicia S. Parkera
aUniversity of Texas Health at San Antonio, San Antonio, TX, USA
bCorresponding Author: Paola Martinez, University of Texas Health at San Antonio, 7703 Floyd Curl Drive, MC 7883, San Antonio, Texas 78229, USA
Manuscript submitted March 13, 2020, accepted April 9, 2020, published online May 30, 2020
Short title: Unsteady Gait Causing Reversible Weakness
doi: https://doi.org/10.14740/jnr582
| Abstract | ▴Top |
In this manuscript, we highlight the importance of human immunodeficiency virus (HIV) testing in patients presenting with a clinical picture suggestive of amyotrophic lateral sclerosis (ALS), particularly when there are other findings not consistent with a primary motor neuron disease.
Keywords: Clinical neurology; Clinical neurology examination; HIV; Post-infectious; Amyotrophic lateral sclerosis
| Introduction | ▴Top |
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder of unknown cause, which can be mimicked by a wide variety of secondary diseases. Given ALS is currently untreatable with a greater than 90% 5-year mortality rate, identification of any potentially treatable mimic condition is paramount [1]. We present a case which highlights the importance of further investigation when clinical or radiographic findings are not entirely consistent with a primary motor neuron disease.
| Case Report | ▴Top |
A 31-year-old man presented to the emergency department with a 3-week history of progressive lower extremity weakness, numbness and gait requiring the use of a cane. The onset of symptoms began with right leg cramping episodes lasting less than 1 min not at any particular time. He reported previously normal gait and strength. He had two falls due to unsteadiness and was reporting trouble stopping once gait was initiated. He had to quit his job as a server due to his gait impairment. He stated that he had been taking magnesium and vitamin B6 over the counter to help with leg cramping with mild improvement. He was admitted to neurology service for workup.
The patient’s examination was notable for intact mentation and cranial nerves, motor exam with 5/5 strength in bilateral upper extremities and left lower extremity, and 4/5 in right lower extremity. Tone was severely increased in bilateral lower extremities, and reflexes were 2+ in biceps, brachioradialis, and triceps, and 3+ in patella with equivocal plantar responses. Decreased temperature to mid shin bilaterally and decreased vibration and proprioception at the great toes were found. There was no dysmetria. Magnetic gait with small shuffling steps, dystonia on the right and retropulsion were noted.
Patient’s initial lab work was remarkable for an elevated erythrocyte sedimentation rate (ESR) (85 mm/h); however, C-reactive protein (CRP) was within normal limits. Total protein was elevated at 9.5 mg/dL, albumin was within normal limits and increased gamma globulins were found on serum protein electrophoresis (SPEP). Mild leukopenia was found (white blood cell (WBC) count of 3,260). Patient’s human immunodeficiency virus (HIV)-1 initial screen was positive, which was confirmed by Western Blot. His viral load was at 375,000 copies with absolute CD4 count less than 40 cells. His cerebrospinal fluid had normal values for his cell count, protein and glucose; however, there were reactive lymphocytes. Cerebrospinal fluid (CSF) gram stain and meningoencephalitis polymerase chain reaction (PCR) panel were negative. CSF was positive for oligoclonal bands, and his myelin basic protein was elevated (31 ng/mL) indicating central nervous system (CNS) inflammation and myelin breakdown respectively. John Cunningham (JC) virus enzyme-linked immunosorbent assay (ELISA) (STRATIFY JCV) was positive, indicating that patient has been exposed to JC virus in the past. The JC virus quantification test reported less than 10 copies per mL and was thus negative for active infection.
Brain magnetic resonance imaging (MRI) revealed patchy and confluent T2 hyperintensities within the bilateral frontal periventricular and deep white matter, involving the genu of the corpus callosum. There were foci of restricted diffusion within the deep white right frontal lobe, right frontal centrum semiovale and left frontal periventricular white matter. The bilateral corticospinal tracts were hyperintense on T2 from the posterior limb of the internal capsule to the cerebral peduncles. There was asymmetrically right more than left parietal lobe atrophy, superior vermis atrophy and ex vacuo dilatation. There were no contrast-enhancing lesions (Fig. 1). Regarding spinal imaging, MRI of cervical spine (C-spine) was within normal limits. MRI of thoracic spine (T-spine) showed multifocal abnormal signal at T7 and within the right lateral T10/T11 cord, which was not contrast enhancing.
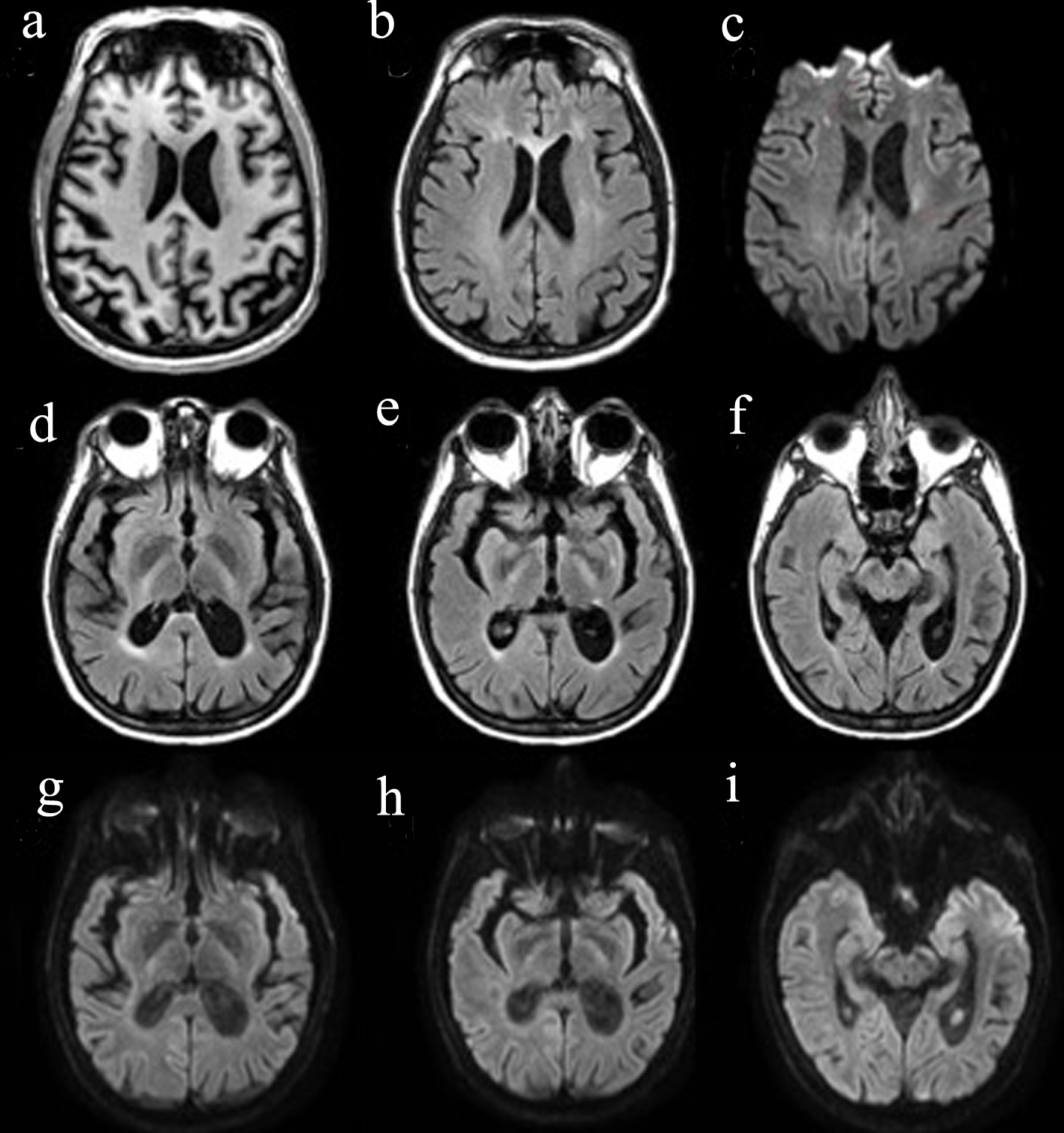 Click for large image | Figure 1. Brain MRI. (a) T1 image showing brain atrophy, more pronounced on the L side; (b) T2 FLAIR demonstrating the hyperintense genu of the corpus callosum, as well as scattered periventricular patchy and confluent white matter hyperintensities; (c) DWI showing diffusion restriction in the deep white matter; (d-f) T2 FLAIR visualizing T2 hyperintensities along the corticospinal tract from the internal capsule to the cerebral peduncles; (g-i) DWI showing subtle diffusion restriction along the corticospinal tract from the internal capsule to the cerebral peduncles. FLAIR: fluid-attenuated inversion recovery; DWI: diffusion weighted imaging; MRI: magnetic resonance imaging. |
Nerve conduction studies were notable for mild sensory-motor demyelinating peripheral polyneuropathy, with a mild degree of axonal loss, affecting his lower limbs. Electromyography showed diffuse fasciculation potentials in upper and lower limbs, with the finding of denervation potentials in lower limbs. There were no fasciculation potentials in the bulbar muscles. The findings of diffuse fasciculation potentials in multiple limb muscles with denervation potentials in the lower limbs were concerning for motor neuron disease [1, 2].
| Discussion | ▴Top |
The laboratory results established a new diagnosis of HIV infection of unknown chronicity, though given the high viral load HIV was likely present for months to years prior to presentation. This information broadened the differential to a considerable variety of neurological manifestations. Etiology of these disorders is multifactorial and not well understood, and potential mechanisms comprise both the immune-mediated effects of the virus and the conditions that arise due to HIV infection. Consequently, HIV-associated neurological disease can be split into two broad categories [3]. The first category is those related to primary HIV infection itself, such as HIV-associated neurocognitive disorder (HAND), myelopathy, radiculopathy, myopathy, or peripheral neuropathies. The second comprises those arising as a result of long-standing infection, such as opportunistic meningoencephalitis (as well as fungal infections, toxoplasmosis, etc.), progressive multifocal leukoencephalopathy, and malignancies such as CNS lymphoma. However, this can be unpredictable, with many of these manifestations seen at any stage of the disease. Fortunately, our patient’s diagnostic workup was negative for active secondary infection.
Based on the electromyogram (EMG) results (diffuse fasciculations and denervation potential in lower limbs) and imaging (MRI showing T2 hyperintensities and diffusion restriction of bilateral corticospinal tracts) in the setting of HIV-1 infection, with negative workup for opportunistic infections, it is possible that our patient’s presentation represented ALS or a reversible ALS-like syndrome associated or precipitated by HIV-1 infection [4].
Asymmetric lower extremity weakness localizes to either the primary motor cortex, spinal cord corticospinal tract below T1, or peripheral nerves supplying the leg. The increased tone of his bilateral lower extremities with the increased bilateral lower extremity reflexes in a subacute presentation helps localize to an upper motor neuron process [5]. Painful cramps could be attributed to his severe increased tone or a lower motor neuron process. His symmetric decreased sensation to temperature, vibration and proprioception to bilateral distal extremities suggests distal polyneuropathy versus less likely a spinal cord lesion, given the bilateral loss of vibration and proprioception, unilateral corticospinal cord involvement and bilateral spinothalamic involvement [5]. Lack of sensory loss in no specific distribution makes a peripheral nerve lesion less likely. Gait with small shuffling steps, narrow-based, magnetic, with apraxia would point to a frontal gait affecting the frontal lobes or frontal subcortical white matter. Unsteadiness with retropulsion would suggest substantia nigra or basal ganglia localization.
Differential diagnosis can be separated into cortical, myelopathic and pathologies that involve both. Cortical lesions include watershed infarctions, neoplasms, infectious diseases such as PML, inflammatory, autoimmune encephalitis or vasculitis. Myelopathic pathologies also include neoplastic or paraneoplastic cord lesion, infectious diseases or post-infectious myelopathy, trauma with injury to anterior cord, transverse myelitis, and anterior spinal artery infarction [6, 7]. Other possibilities include multiple sclerosis, hereditary spastic paraplegia, thyrotoxic myopathy, multifocal motor neuropathy, B12 deficiency, and lead toxicity [8].
It was also considered that patient could have a component of HIV myelopathy (based on T2 hyperintensities in his thoracic spine MRI), as well as HIV-associated neuropathy (nerve conduction study (NCS) showing a sensory-motor mostly demyelinating polyneuropathy) and HIV encephalopathy (as patient had cerebral volume loss and diffuse T2 hyperintensities in the deep white matter) [9]. However, the EMG findings of sensory nerve involvement and MRI findings of corticospinal tract hyperintensities cannot be explained by myelopathy, HIV encephalopathy or pure neuropathy [1, 9, 10].
Conclusions
As in our case, HIV infection can result in a clinically ALS-like syndrome [1, 8]. Potentially differentiating factors include onset at a younger age than is typical in ALS, and as previously mentioned, the presence of other neurological sequelae of HIV, such as myelopathic findings or peripheral sensory neuropathy [1, 4]. There are case reports in HIV-associated ALS-like syndromes of imaging findings such as T2 hyperintense lesions in spinal cord gray matter [8, 11]. There was significant variability in CD4 count, serum viral load or even CSF viral load, indicating that these studies do not always correlate with the risk of developing neurological complications, or with their severity [10]. The exact pathophysiology of corticospinal tract dysfunction is not known; however, it is believed to be a “parainfectious” effect on the spinal cord motor neurons [10, 12]. Alternate hypotheses include activation of endogenous retroviruses, or micro-infarctions due to hypercoagulable states [9].
The established treatment for these complications is initiation of highly active antiretroviral therapy (HAART) therapy, after ruling out co-existing opportunistic infections that could be contributing to the patient’s presentation [1, 8]. There are some rare cases of the use of immune-modulating therapies such as intravenous immunoglobulin (IVIG) or plasmapheresis resulting in clinical improvement or stabilization, further supporting the aforementioned immune-mediated theory [10]. While there are reports of HAART initiation resulting in improvement or even reversal of neurological manifestations, the response is variable, and some patients can develop neurological complications even long after commencement of HAART [1, 13].
Nevertheless, this case highlights the importance of HIV testing in patients presenting with symptoms suggestive of ALS, particularly when there are clinical or radiographic findings that are not consistent with a primary motor neuron disease [2]. Although treatment efficacy is variable, it remains a potentially reversible ALS syndrome mimic [2, 8, 11]. In our case, at 1-month follow-up the patient’s lower leg strength was found to be improved.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Subject has given verbal informed consent.
Author Contributions
PM, JD, HL, and KS contributed to design and conceptualization of study, data collection and analysis, drafting and revision of manuscript. AP supervised project, design and conceptualization of study, and revised the manuscript for intellectual content.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Bowen LN, Tyagi R, Li W, Alfahad T, Smith B, Wright M, Singer EJ, et al. HIV-associated motor neuron disease: HERV-K activation and response to antiretroviral therapy. Neurology. 2016;87(17):1756-1762.
doi pubmed - Rowland LP. What's in a name? Amyotrophic lateral sclerosis, motor neuron disease, and allelic heterogeneity. Ann Neurol. 1998;43(6):691-694.
doi pubmed - Gray F, Chretien F, Vallat-Decouvelaere AV, Scaravilli F. The changing pattern of HIV neuropathology in the HAART era. J Neuropathol Exp Neurol. 2003;62(5):429-440.
doi pubmed - Shimojima Y, Yazaki M, Kaneko K, Fukushima K, Morita H, Hashimoto T, Ikeda S. Characteristic spinal MRI findings of HIV-associated myelopathy in an AIDS patient. Intern Med. 2005;44(7):763-764.
doi pubmed - Ravits J, Paul P, Jorg C. Focality of upper and lower motor neuron degeneration at the clinical onset of ALS. Neurology. 2007;68(19):1571-1575.
doi pubmed - Robinson-Papp J, Simpson DM. Neuromuscular diseases associated with HIV-1 infection. Muscle Nerve. 2009;40(6):1043-1053.
doi pubmed - Gordon PH, Cheng B, Katz IB, Mitsumoto H, Rowland LP. Clinical features that distinguish PLS, upper motor neuron-dominant ALS, and typical ALS. Neurology. 2009;72(22):1948-1952.
doi pubmed - Ghasemi M. Amyotrophic lateral sclerosis mimic syndromes. Iran J Neurol. 2016;15(2):85-91.
- Chen H, Lin F, Liu S, Da Y, Guo D. Neurological manifestations, laboratory and neuroimaging features in HIV-infected patients. Neurosciences (Riyadh). 2017;22(4):311-315.
doi pubmed - Bogoch, II, Wilson MR, Chad DA, Venna N. Acute lower motor neuron syndrome and spinal cord gray matter hyperintensities in HIV infection. Neurol Neuroimmunol Neuroinflamm. 2015;2(4):e113.
doi pubmed - Di Rocco A. Diseases of the spinal cord in human immunodeficiency virus infection. Semin Neurol. 1999;19(2):151-155.
doi pubmed - Santosh CG, Bell JE, Best JJ. Spinal tract pathology in AIDS: postmortem MRI correlation with neuropathology. Neuroradiology. 1995;37(2):134-138.
doi pubmed - Skiest DJ. Focal neurological disease in patients with acquired immunodeficiency syndrome. Clin Infect Dis. 2002;34(1):103-115.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.
