| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website http://www.neurores.org |
Original Article
Volume 2, Number 3, June 2012, pages 82-87
Changes in Peripheral Blood Mononuclear Cells-Novel Evidence for an Immunomodulatory Aspect in Bell’s Palsy?
Stefan S. Kassnera, d, Sarah Schoettlera, Gabriel A. Bonaterrab, Anne Fabera, Karl Hormanna, Ulrich R. Goesslera, Ralf Kinscherfb, c, Jens Stern-Straetera, c
aDepartment of Otorhinolaryngology, Head and Neck surgery, University Hospital Mannheim, Theodor-Kutzer-Ufer 1-4, Mannheim, Germany
bAnatomy and Cell Biology, Department of Medical Cell Biology, Robert-Koch-Strasse 8, 35032 Marburg, Germany
cThese authors contributed equally to this work
dCorresponding author: Stefan S. Kassner
Manuscript accepted for publication June 17, 2012
Short title: Changes in Peripheral Blood Mononuclear Cells
doi: https://doi.org/10.4021/jnr108w
| Abstract | ▴Top |
Background: Patients with Bell’s Palsy (BP): a very common neuropathy of the facial nerve-showed significant decreases of T- and B-lymphocytes in earlier studies. Besides a viral infection an autoimmune process leading to this disease has been discussed.
Methods: 15 patients with BP and 15 healthy, age-matched individuals were included in this study. In addition to routine blood parameters peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient. Afterwards, in CD14+ (monocytes), CD68+ (macrophages), CD3+ (T-lymphocytes) or CD19+ (B-lymphocytes) the percentage of cells, expressing pro-inflammatory (CD40, TNF-α, and COX-2), pro-apoptotic (Caspase-3, PARP), pro-adhesive (CD38) and oxidative stress-related (MnSOD) proteins were measured by two-color fluorescence-activated cell sorter analyses.
Results: In comparison to healthy individuals, patients with BP revealed significantly elevated plasma levels of C-reactive protein and total leukocyte count, while levels of T- and B-lymphocytes were significantly decreased. In BP patients all subpopulations under test showed a significant increase in percentage of CD38+ as well as of pro-inflammatory (CD40+, TNF-α+) cells; additionally monocytes showed a significant increase in percentage of MnSOD positive cells.
Conclusions: Our data shows new evidence for an involvement of the immune system in BP. Therefore, we suggest that future investigations of PBMCs in BP patients represent a very promising approach to further elucidate possible pathomechanisms.
Keywords: Bell’s Palsy; Inflammation; CD40; TNF-α; PBMC; CRP; CD38; Adhesion; Lymphocytes
| Introduction | ▴Top |
Bell’s Palsy (BP) is a very common neuropathy of the facial nerve. The processes leading to the disease remain unclear [1]. Several years ago, cellular autoimmune mechanisms in the pathogenesis of BP have been suggested to be involved [2, 3]. However, novel data reveal that treatment with corticosteroids with or without an antiviral drug is recommended, suggesting an involvement of immunomodulatory processes [4, 5].
Moreover, earlier studies showed a significant decrease in levels of T- and B-lymphocytes in BP patients [6, 7] supporting the potential pathogenetic relevance of these cells. Furthermore a decreased level of antibody producing CD19+ B-lymphocytes has been discussed to cause an insufficient clearance of neurotropic viruses or virus particles possibly responsible for disease induction and/or progression [8]. In this context, different neurotropic viruses e.g. herpes simplex virus type 1 (HSV-1) have been proposed to be a possible disease inductor, due to the fact that HSV-1 DNA could be detected in endoneural fluid and saliva from BP patients [9, 10]. Additionally, data of an animal model revealed, that a reactivation of a HSV-1-infection in combination with immune suppression induce BP, indicating the special importance of the immune system in this disease and especially the cellular system responsible for viral defense-the lymphocytes [11].
Most recently we have shown an elevated expression of pro-inflammatory, pro-adhesive and pro-apoptotic genes/proteins in patients with acute stroke, Alzheimer dementia, sudden sensorineural hearing loss or vestibular neuritis [12-15]. In these diseases, the expression pattern was totally different among the patient groups suggesting a distinct involvement of special peripheral blood mononuclear cell (PBMC) subpopulations (monocytes, macrophages, T- or B-lymphocytes) in disease induction and/or progression. We hypothesize that BP patients have elevated levels of PBMCs expressing pro-inflammatory and adhesion-relevant proteins as well as increased pro-inflammatory biochemical plasma parameters in comparison with matched control subjects. Because of increasing existing evidence of an involvement of PBMC in the processes of BP, our present study seemed to be a very promising approach.
| Material and Methods | ▴Top |
Subjects
15 patients with BP aged from 18 - 59 years, hospitalized at the Department of Otolaryngology, Head and Neck Surgery (University Hospital of Mannheim, Germany) and 15 age-matched healthy subjects (= control) were enrolled in this study. Patients and control subjects with chronic inflammatory diseases (e.g. diabetes) as well as smokers were excluded from this study. BP was diagnosed by clinical examination and classified by House Brackmann Facial Nerve Grading System [14]. All included subjects showed a moderate or moderately severe loss of function, classified as a House Brackmann Score III or IV. The interval between the onset of symptoms and blood collection varied between 6 to 24 hours. All patients received treatment with corticosteroids after collection of blood samples. All patients and subjects with regular anti-inflammatory treatment such as non-steroidal anti-inflammatory drugs (NSAID, corticosteroids etc.) before onset of symptoms were excluded from this study. The study was approved by the local ethics committee of the University Hospital of Mannheim. Additionally, informed written consent was provided.
Blood samples, blood count and serologic testing
Blood samples were routinely taken from the cubital vein. C-reactive protein (CRP) levels and blood count were quantified by standard methods at the laboratory of the University Medical Hospital Mannheim. Serologic testing included antibody titers against varicella zoster, herpes simplex and borreliosis. Patients with an acute or reactivated infection of one of these diseases indicated by elevation of IgM- or IgG-levels above the reference levels were excluded from the study.
Isolation of PBMCs
PBMCs were isolated by density gradient (Biocoll, (1.077 g/mL) Biochrom AG, Berlin, Germany) according to the manufacturer’s specifications, as previously described [15]. After collection, PBMCs were washed twice with endotoxin-free PBS without Ca2+ and Mg2+ (PAA Laboratories GmbH, Pasching, Austria).
Fluorescence Activated Cell Sorter (FACS) analysis
Isolated PBMCs were fixed (5 min, RT) with 4% paraformaldehyde (Sigma Chemical Co., St. Louis, USA) and washed three times with PBS. Cells were resuspended in 100 µL PBS/0.1% BSA. For detection of intracellular localized antigens, permeabilization was performed with 0.1% saponin, 10 min, RT (Carl Roth GmbH and Co KG, Karlsruhe, Germany).
FACS analysis were performed with aliquots of 5 x 105 fixed cells using the following fluorescein-5-isothiocyanate (FITC) or phycoerythrin (PE) conjugated monoclonal (mAb) or polyclonal (pAb) anti-human antibodies directed against: CD40-Receptor (LINARIS GmbH, Wertheim-Bettingen, Germany), CD38 (EXBIO, Praha, Czech Republic), TNF-α (LINARIS), cyclooxygenase-2 (COX-2, Exalpha Biologicals Inc., Shirley, USA), Poly(ADP-ribose)-Polymerase 1 (PARP, BD Biosciences Heidelberg, Germany), MnSOD (Manganese-Superoxide-Dismutase, Bender MedSystems GmbH, Vienna, Austria), caspase-3 (BD Biosciences Pharmingen) and for staining of subpopulations CD14+ (monocytes and macrophages), CD68+ (macrophages), CD3+(T-lymphocytes) or CD19+ (B-lymphocytes) (LINARIS).
Two-color flow cytometric analyses were performed using a BD FACS Canto II six-color flow cytometer and BD FACSDiva software (BD Biosciences). The gate was set around the PBMCs to exclude other cells or debris from analysis. Routinely 10,000 cells per tube were counted. The isotype IgG was used for background control. The results were expressed as percentage of total PBMCs in suspension, and the data converted into frequency histograms and dot plots.
Statistical methods
Results are presented as mean ± standard error of the mean (SEM). Data of patients and control persons were compared by the Mann Whitney U or by the unpaired Student’s t-test, using the SIGMASTAT 3.1 program (Jandel Scientific, Erkrath, Germany). A P value of 0.05 or less was chosen for statistical significance.
| Results | ▴Top |
Patient characteristics and blood parameters
CRP level was significantly (P = 0.04) 2.5-fold increased, as well as the amount of leukocytes, which showed a significant (P = 0.008) 1.3-fold increase in patients with BP compared to healthy controls (Table 1). Differential blood count analysis including leukocyte subpopulations showed significant decreases of percentages of lymphocytes and eosinophils in BP patients in comparison to healthy subjects there was a significant increase in percentage of neutrophils (Table 1). In particular we found a 1.2-fold increase in percentage of neutrophils (P = 0.026), a-1.8-fold decrease in percentage of eosinophils (P = 0.003) and a 1.3-fold decrease in percentage of lymphocytes (P = 0.033) (Table 1).
 Click to view | Table 1. Age, Blood Lipids, Number of Leukocytes (Including Subpopulations) and PBMC in BPPatients and Healthy Subjects |
PBMC subpopulations and expression of pro-inflammatory (TNF-α, COX-2), pro-apoptotic (caspase-3 and PARP), adhesion-relevant (CD38) or oxidative stress related (MnSOD) proteins
According to the finding of the differential blood count, the percentage of T-lymphocytes (CD3+ cells) as well as B-lymphocytes (CD19+ cells) was significantly (P= 0.005 and P = 0.007) 1.3-fold and 1.4-fold decreased in patients with BP compared to healthy controls (Fig. 1a), while levels of CD14+ monocytes and CD68+macrophages were similar in BP patients compared to healthy controls (Fig. 1a).
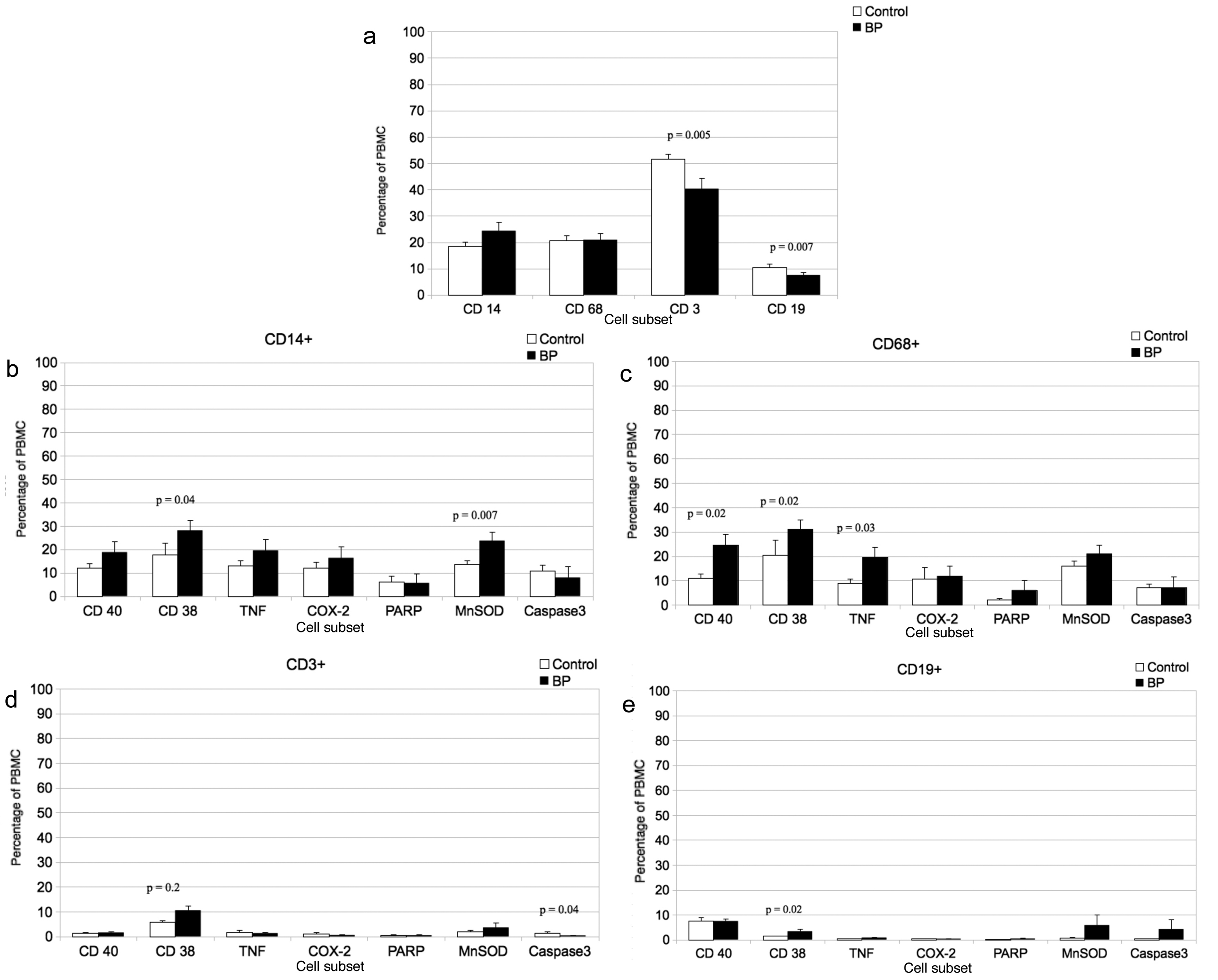 Click for large image | Figure 1. a. Percentage of CD14 (monocytes), CD68 (macrophages), CD3 (T-lymphocytes and CD19 (B-lymphocytes) immunoreactive PBMCs in BP patients and healthy subjects. Mean ± SEM; n = 15; P ≤ 0.05 (significance). b. Percentage of double-immunostained CD19 (B-lymphocytes) positive and CD40, TNF-α, COX-2, (proinflammatory markers), PARP and Caspase-3 (proapoptotic markers) or CD38 (adhesion relevant marker) immunoreactive PBMCs in BP patients and healthy subjects (control). Mean ± SEM; n = 15; P ≤ 0.05 (significance). c. Percentage of double-immunostained CD3 (T-lymphocytes) positive and CD40, TNF-α, COX-2, (proinflammatory markers), PARP and Caspase-3 (proapoptotic markers) or CD38 (adhesion relevant marker) immunoreactive PBMCs in BP patients and healthy subjects (control). Mean ±SEM; n = 15; P ≤ 0.05 (significance). d. Percentage of double-immunostained CD68 (macrophages) positive and CD40, TNF-α, COX-2, (proinflammatory markers), PARP and Caspase-3 (proapoptotic markers) or CD38 (adhesion relevant marker) immunoreactive PBMCs in BP patients and healthy subjects (control). Mean ± SEM; n = 15; P ≤ 0.05 (significance). e. Percentage of double-immunostained CD14 (monocytes) positive and CD40, TNF-α, COX-2, (proinflammatory markers), PARP and Caspase-3 (proapoptotic markers) or CD38 (adhesion relevant marker) immunoreactive PBMCs in BP patients and healthy subjects (control). Mean ± SEM; n = 15; P ≤ 0.05 (significance). |
In the monocyte subpopulation (CD14+) of patients we found a significantly 1.6-fold increase of CD38 and a significantly 1.7-fold increase of MnSOD positive cells (P =0.04 and P = 0.007) compared to healthy subjects (Fig. 1b). However, in this subpopulation there was no significant difference in percentage of CD40+, TNF-alpha+, COX-2+, PARP+ or caspase 3+ cells of BP patients compared to healthy controls (Fig. 1b).
On the other hand macrophages of BP patients showed significantly increased percentage of CD40+ (2.2-fold; P = 0.02), CD38+ (1.6-fold; P = 0.02) and TNF-alpha+ (2.2-fold; P = 0.03) positive cells compared to healthy subjects (Fig. 1c).
The T-lymphocyte (CD3+) subpopulation of BP patients showed a significant (P =0.02) 1.8-fold increase in percentage of CD38+ cells, whereas the percentage of caspase-3+ positive cells was significantly decreased (3.5-fold; P = 0.04) compared to controls (Fig. 1d). However, in the T-lymphocyte subpopulation, the percentages of cells, expressing other pro-inflammatory, pro-apoptotic or oxidative stress proteins, like TNF-alpha, COX-2, PARP, MnSOD, or CD40 showed no significant differences between BP patients and healthy subjects (Fig. 1d).
Finally, CD19+ B-lymphocytes of BP patients, showed a significant (P = 0.02) 2.3-fold increased percentage of CD38+ cells compared to healthy subjects (Fig. 1e). However, in the B-lymphocyte subpopulation, the percentages of cells, expressing pro-inflammatory, pro-apoptotic or oxidative stress proteins showed no significant differences between BP patients and healthy subjects (Fig. 1e).
| Discussion | ▴Top |
Immunophenotyping has recently been described to be a valuable effort to find new immunomodulatory changes in patients with uncharacteristic inflammatory diseases [16]. In this context, we found significantly increased levels of CRP and leukocytes in patients with BP compared to healthy subjects. Even though these elevations do not reach a pathological range, as during e. g. bacterial infection, which has been recently hypothesized to be related to BP [17], they can be interpreted as a sign for inflammatory processes being involved in this disease. Furthermore, we showed that levels of B- and T-lymphocytes were significantly decreased. These results confirm and extend data of others [18]: Several studies have shown, that the absence of B cells can lead to exacerbated pathological inflammatory responses in autoimmune diseases [19], just as the regulatory T cells play an important role in the maintenance of self-tolerance since depletion of these cells elicits autoimmune diseases [20], this diminution observed could be associated with an immunosuppressive condition by BP patients. Additionally, in both of these subpopulations (T- and B-lymphocytes) we found a significantly elevated percentage of pro-adhesive CD38+ cells. This seems to be of major interest, because CD38 is known as a crucial receptor to initiate adhesion and transmigration of leukocytes via its counter receptor CD31 [21]. The immediate tansmigration of immunocompetent cells into the CNS is an important step to initiate inflammatory processes in the brain-vice versa while its activity could be inhibited by blocking the CD31 molecule [22]. In this context, it is necessary to mention that we found similar changes in patients with sensorineural hearing loss, where an involvement of immunocompetent cells and their transmigration from peripheral blood in the inner ear is also proposed by us and others [15, 23, 24]. Thus, a pivotal role of PBMC in BP disease may also be suggested. In this context, we found a significant increase in percentage of TNF-alpha and CD40 positive cells in the macrophage subpopulation of patients with BP compared to healthy subjects. TNF-alpha is known to influence B-Cell differentiation and immunoglobulin-production [25]. Additionally, an elevated expression of CD40 on lymphocytes as well as brain endothelial cells has been described to regulate cellular adhesion and transmigration [26]. Thus, our findings could propose an elevated activity of T- and especially B-lymphocytes in BP leading to an increased extravasation of these cells, reflected by the significant decrease of lymphocytes in patients with BP compared to healthy subjects and not an immunosuppression with the concomitant reduction in the T and B-lymphocyte subpopulation.
A concrete trigger and the molecular mechanisms of the disease remain yet unknown. Moreover, while an edema of the facial nerve has longer been discussed, recent studies suggest an asymmetry between the right and left middle part of the labyrinthine portion to be responsible for a higher risk of developing BP [27]. Only prednisolone has been described to significantly increase clinical recovery of paresis [28]. Like in other neurological and neurotological diseases in which the pathogen is unknown, a viral infection was suggested to be responsible for disease induction, because, e.g. HSV DNA could be found in endoneurial fluid and muscle of patients with BP [10]. However, new meta-analysis showed no clinical improvement when using antivirals plus corticosteroids compared to steroids alone [29]; thus, unfortunately the theory of a viral infection is not profoundly endorsed.
The PBMC subpopulation of macrophages showed a significantly increased percentage of CD40+ cells while in all subpopulations percentage of CD38+ cells was significantly elevated, too. So while the potential for extravasation seems to be shared by all subpopulation, the potential for inflammatory changes seems to be focused on the macrophage subpopulation.
According to our data additional therapeutical approaches should focus on the CD40 cascade, on the one hand inhibiting its inflammatory impact and, on the other hand, the pro-adhesive aspect mediated by CD38+, which may be responsible for the accumulation of these pro-inflammatory cells close to the facial nerve leading to initiation and/or progression of inflammation, which consecutively cause the swelling of the nerve with consequences for the clinical symptomatic.
Further studies are needed to clarify, whether inhibition of inflammatory processes in the blood of BP patients may improve the recovery of the disease and hence, the efficacy of clinical treatment and outcome.
Our data show new evidence for an involvement of the immune system in BP by monitoring a pro-inflammatory and pro-adhesive activation of PBMC in these patients. Since we found a significant decrease in levels of lymphocytes, an extravasation of proinflammatory PBMC could contribute to progression of BP and should therefore be subject of further investigations. Additionally, therapeutic intervention should address the improvement of BP symptoms with special respect to alterations of percentage of pro-inflammatory and pro-adhesive protein expressing PBMC subpopulations.
Further studies in this context are promising to address the expressions of the relevant genes to elucidate possible pathomechanisms and their potential use to monitor BP therapy.
Acknowledgments
The authors would like to thank Mrs. Petra Prohaska and Mrs. Ulrike Traut for their technical assistance.
Abbreviations
BP: Bell’s Palsy; CD: cluster of differentiation; COX-2: cyclooxygenase-2; CPP32: caspase-3; CRP: C-reactive protein; DNA: deoxyribonucleic acid; HDL: high density lipoprotein; HSV1: herpes simplex virus type 1; LDL: low-density lipoproteins; PARP: poly(ADP-ribose) polymerase; PBMCs: peripheral blood mononuclear cells; TNF-α: tumor necrosis factor-alpha
| References | ▴Top |
- Devriese PP, Fidler VJ. Atmospheric pressure and idiopathic facial paralysis (Bell's palsy). Aviat Space Environ Med. 1977;48(7):672-673.
pubmed - McGovern FH, Estevez J, Jackson R. Immunological concept for Bell's palsy: further experimental study. Ann Otol Rhinol Laryngol. 1977;86(3 Pt 1):300-305.
pubmed - Abramsky O, Webb C, Teitelbaum D, Arnon R. Cellular immune response to peripheral nerve basic protein in idiopathic facial paralysis (Bell's palsy). J Neurol Sci. 1975;26(1):13-20.
pubmed doi - Lagalla G, Logullo F, Di Bella P, Provinciali L, Ceravolo MG. Influence of early high-dose steroid treatment on Bell's palsy evolution. Neurol Sci. 2002;23(3):107-112.
pubmed doi - Axelsson S, Lindberg S, Stjernquist-Desatnik A. Outcome of treatment with valacyclovir and prednisone in patients with Bell's palsy. Ann Otol Rhinol Laryngol. 2003;112(3):197-201.
pubmed - Aviel A, Ostfeld E, Burstein R, Marshak G, Bentwich Z. Peripheral blood T and B lymphocyte subpopulations in Bell's palsy. Ann Otol Rhinol Laryngol. 1983;92(2 Pt 1):187-191.
pubmed - Gorodezky C, Carranza JM, Bustamante A, Yescas P, Martinez A, Alonso Vilatela ME. The HLA system and T-cell subsets in Bell's palsy. Acta Otolaryngol. 1991;111(6):1070-1074.
pubmed doi - Bergmann CC, Ramakrishna C, Kornacki M, Stohlman SA. Impaired T cell immunity in B cell-deficient mice following viral central nervous system infection. J Immunol. 2001;167(3):1575-1583.
pubmed - Furuta Y, Fukuda S, Chida E, Takasu T, Ohtani F, Inuyama Y, Nagashima K. Reactivation of herpes simplex virus type 1 in patients with Bell's palsy. J Med Virol. 1998;54(3):162-166.
pubmed doi - Murakami S, Mizobuchi M, Nakashiro Y, Doi T, Hato N, Yanagihara N. Bell palsy and herpes simplex virus: identification of viral DNA in endoneurial fluid and muscle. Ann Intern Med. 1996;124(1 Pt 1):27-30.
pubmed - Takahashi H, Hitsumoto Y, Honda N, Hato N, Mizobuchi M, Murakami S, Kisaki H, et al. Mouse model of Bell's palsy induced by reactivation of herpes simplex virus type 1. J Neuropathol Exp Neurol. 2001;60(6):621-627.
pubmed - Kassner SS, Bonaterra GA, Kaiser E, Hildebrandt W, Metz J, Schroder J, Kinscherf R. Novel systemic markers for patients with Alzheimer disease? - a pilot study. Curr Alzheimer Res. 2008;5(4):358-366.
pubmed doi - Kassner SS, Kollmar R, Bonaterra GA, Hildebrandt W, Schwab S, Kinscherf R. The early immunological response to acute ischemic stroke: differential gene expression in subpopulations of mononuclear cells. Neuroscience. 2009;160(2):394-401.
pubmed doi - Kassner SS, Schottler S, Bonaterra GA, Stern-Straeter J, Hormann K, Kinscherf R, Gossler UR. Proinflammatory activation of peripheral blood mononuclear cells in patients with vestibular neuritis. Audiol Neurootol. 2011;16(4):242-247.
pubmed doi - Kassner SS, Schottler S, Bonaterra GA, Stern-Strater J, Sommer U, Hormann K, Kinscherf R, et al. Proinflammatory and proadhesive activation of lymphocytes and macrophages in sudden sensorineural hearing loss. Audiol Neurootol. 2011;16(4):254-262.
pubmed doi - House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2):146-147.
pubmed - Janols H, Bredberg A, Thuvesson I, Janciauskiene S, Grip O, Wullt M. Lymphocyte and monocyte flow cytometry immunophenotyping as a diagnostic tool in uncharacteristic inflammatory disorders. BMC Infect Dis. 2010;10:205.
pubmed - Tekgul H, Polat M, Serdaroglu G, Ikizoglu T, Yalaz M, Kutukculer N, Gokben S. Lymphocyte subsets in Bell's palsy: immune pathogenesis and outcome prediction. Pediatr Neurol. 2004;31(4):258-260.
pubmed doi - Mauri C, Ehrenstein MR. The 'short' history of regulatory B cells. Trends Immunol. 2008;29(1):34-40.
pubmed doi - Fillatreau S, Gray D, Anderton SM. Not always the bad guys: B cells as regulators of autoimmune pathology. Nat Rev Immunol. 2008;8(5):391-397.
pubmed doi - Deaglio S, Morra M, Mallone R, Ausiello CM, Prager E, Garbarino G, Dianzani U, et al. Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an Ig superfamily member. J Immunol. 1998;160(1):395-402.
pubmed - Qing Z, Sandor M, Radvany Z, Sewell D, Falus A, Potthoff D, Muller WA, et al. Inhibition of antigen-specific T cell trafficking into the central nervous system via blocking PECAM1/CD31 molecule. J Neuropathol Exp Neurol. 2001;60(8):798-807.
pubmed - Tomiyama S, Jinnouchi K, Ikezono T, Pawankar R, Yagi T. Experimental autoimmune labyrinthitis induced by cell-mediated immune reaction. Acta Otolaryngol. 1999;119(6):665-670.
pubmed doi - Stearns GS, Keithley EM, Harris JP. Development of high endothelial venule-like characteristics in the spiral modiolar vein induced by viral labyrinthitis. Laryngoscope. 1993;103(8):890-898.
pubmed doi - Rieckmann P, Tuscano JM, Kehrl JH. Tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6) in B-lymphocyte function. Methods. 1997;11(1):128-132.
pubmed doi - Omari KM, Dorovini-Zis K. CD40 expressed by human brain endothelial cells regulates CD4+ T cell adhesion to endothelium. J Neuroimmunol. 2003;134(1-2):166-178.
pubmed doi - Kefalidis G, Riga M, Argyropoulou P, Katotomichelakis M, Gouveris C, Prassopoulos P, Danielides V. Is the width of the labyrinthine portion of the fallopian tube implicated in the pathophysiology of Bell's palsy?: a prospective clinical study using computed tomography. Laryngoscope. 2010;120(6):1203-1207.
pubmed - Sullivan FM, Swan IR, Donnan PT, Morrison JM, Smith BH, McKinstry B, Davenport RJ, et al. Early treatment with prednisolone or acyclovir in Bell's palsy. N Engl J Med. 2007;357(16):1598-1607.
pubmed doi - Quant EC, Jeste SS, Muni RH, Cape AV, Bhussar MK, Peleg AY. The benefits of steroids versus steroids plus antivirals for treatment of Bell's palsy: a meta-analysis. BMJ. 2009;339:b3354.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.
