| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website https://www.neurores.org |
Review
Volume 13, Number 1, September 2023, pages 12-32
Functional Neurological Disorder: Historical Trends and Urgent Directions
Yadira Velazquez-Rodriqueza, c, Brooke Fehilyb
aScience & Shamanism International, LLC, Wilmington, DE 19808, USA
bSchool of Biomedical Sciences, Faculty of Medicine, Nursing and Health Sciences, Monash University, Clayton, VIC 3800, Australia
cCorresponding Author: Yadira Velazquez-Rodriquez, Science & Shamanism International, LLC, Wilmington, DE 19808, USA
Manuscript submitted July 8, 2023, accepted September 22, 2023, published online September 30, 2023
Short title: Functional Neurological Disorder
doi: https://doi.org/10.14740/jnr754
- Abstract
- Introduction
- Search Strategy and Selection Criteria
- Historical Trends of FND Classification
- Epidemiological and Social Trends
- Outcomes and Prognosis
- Therapy and Management for FND
- Conclusions
- References
| Abstract | ▴Top |
The objective was to identify the gaps in understanding and management of functional neurological disorders (FNDs) that could be negatively impacting its incidence, prevalence, prognosis, and preventive tools. A narrative review was performed to synthetize evidence from multiple fields including genetic, epidemiological, functional neuroimaging and clinical studies, paying close attention to FND historical trends and recurring themes in nomenclature, classification, epidemiology, therapeutic tools, outcomes, prognosis, and pathophysiology. References included in this review were sourced from PubMed, covering January 1, 2000 to June 30, 2022, and from the references of relevant articles. Multiple problems associated with the current status of approach and management of FNDs were identified, including six major knowledge gaps. To overcome such shortfalls, we recommend the collaborative creation of a multi-network management algorithm that integrates all pathophysiological mechanisms involved in FND onset and perpetuation. It is hoped that an integrative model will facilitate the development of a biographically focused, biopsychosocial-spiritual management and preventive protocol, which incorporates key concepts and skills from the fields of neurology, psychiatry, psychology, and physiotherapy. Such comprehensive and concise protocol could be distributed through upskill programs across several medical specialties. Multidisciplinary collaboration is needed to fill current knowledge gaps, with multispecialty teams helping to overcome the deficits in outcomes and prognosis still affecting FND, one of the commonest and most expensive neurological disorders currently affecting humankind.
Keywords: Functional neurological disorders; Conversion disorder; Functional seizures; Psychotherapy; Pathophysiology
| Introduction | ▴Top |
Functional neurological disorder (FND) is caused by dysfunctional structures, and networks within the nervous system, in the presence of normal anatomy and tissue structure, what experts convey as “normal hardware but dysfunctional software”. FND is associated with a large number of neurological symptoms and manifestations, presenting to multiple specialists. Despite clear findings steaming from epidemiological, functional imaging, and clinical studies, FND outcomes and prognosis remain suboptimal. In this manuscript, we review FND historical trends and current epidemiological, pathophysiological, therapeutic, and clinical data. Each section presents scientific evidence, highlighting knowledge gaps and emphasizing current approaches to management of FND, which will be of greatest interests to clinicians encountering FND.
| Search Strategy and Selection Criteria | ▴Top |
References included in this review were identified by searches of PubMed between January 1, 2000 and June 30, 2022, and from the references of relevant articles. The following search terms were used: “hysteria”, “functional”, “functional symptom”, “functional neurological disorder”, “FND”, “conversion disorder”, “somatoform”, “psychogenic”, “psychosomatic”, “medically unexplained”, “functional motor”, “functional weakness”, “functional movement”, “functional seizures”, “functional cognitive”, “PNES”, “nonepileptic seizure”, “functional neurological”, and “children”. There were no language restrictions. The final reference list was generated on the basis of relevance to the topics covered in this review.
| Historical Trends of FND Classification | ▴Top |
Nomenclature throughout history
FND denotes a condition first described in 1900 B.C. in Egypt, characterized by behavioral abnormalities among women who experienced impediments in marriage and childbirth [1, 2]. By 400 B.C., the condition was named “hysteria”, derived from the Latin word “hystericus”, meaning uterus or womb [3]. During the 19th century, Sigmund Freud introduced the term “conversion”, describing it as physical symptoms resulting from childhood trauma and unconscious conflicts, which were converted to neurological symptoms [4].
In 1968, the International Classification of Diseases (ICD)-6, and Diagnostic and Statistical Manual of Mental Disorders (DSM)-II [5] classified hysteria as “psychoneurosis”, or of psychogenic origin. In the DSM-III and ICD-9 of 1980, conversion disorder was categorized under “somatoform disorders”, conjoined with hypochondriasis, and somatization [6]. Past and current nomenclature and classification consistently link subconscious psychological processes to functional physical ailments. The importance of this association is further explored in the pathophysiology section.
In 1994, the DSM-IV noted that conversion disorder had multiple subtypes, encompassing motor, sensory, seizures, and mixed [7]. The DSM-V of 2013 [8] introduced the term functional neurological symptom disorder, between parentheses, representing a pathophysiological understanding extending beyond the psychological theory of conversion. In 2022, the DSM-V-TR (text revision) [9] updated the nomenclature by simply moving “conversion disorder” between parentheses. Thus, “conversion” remains part of the latest classification, despite its affiliation to Sigmund Freud’s pathophysiology, rooted in purely psychological mechanisms of disease production, accompanied by associated stigma.
The official term for FND is “functional neurological symptom disorder”, which is rarely used by scientists, clinicians, or patients. Instead, the term FND, without the word symptom is the most often used by patients and the medical community. In fact, patients with FND rarely present with a single symptom, but rather a multitude of symptoms, spanning diverse organs and systems, frequently simultaneously, changing over time, and oftentimes disabling. This disparity between the DSM’s official nomenclature used in healthcare records, patients’ and clinicians’ experiences, is problematic. The official name “functional neurological symptom disorder”, inadequately encapsulates its clinical presentations. Members of the DSM workgroup should consider adjusting the name of the condition to “functional neurological disorders”, eliminating the word “symptom” and the term “conversion”.
FND will be the terminology utilized throughout this manuscript to align with the predominant body of evidence. Terminologies such as “conversion”, “somatoform”, “psychogenic” and “psychosomatic” will be avoided and substituted with “functional”. For example, “functional seizures” will be used instead of “psychogenic non-epileptic seizures”. We extend this recommendation to all healthcare professionals and patients.
Functional disorders (FD) and FND: current classification considerations
FND refers to a neurological disorder arising from dysfunctional structures and pathways within the nervous system, categorically in the presence of normal anatomy and structure. As the nervous system participates in the functioning of all organs and systems of the human body, its dysfunction can manifest with a multitude of clinical presentations. As a result, patients present to various medical specialists [10] outside the boundaries of neurology (Fig. 1). However, the commonalities underlying the many functional symptoms encountered in medical practices are frequently overlooked, with patients frequently referred to multiple consultants, and often sub-optimally managed.
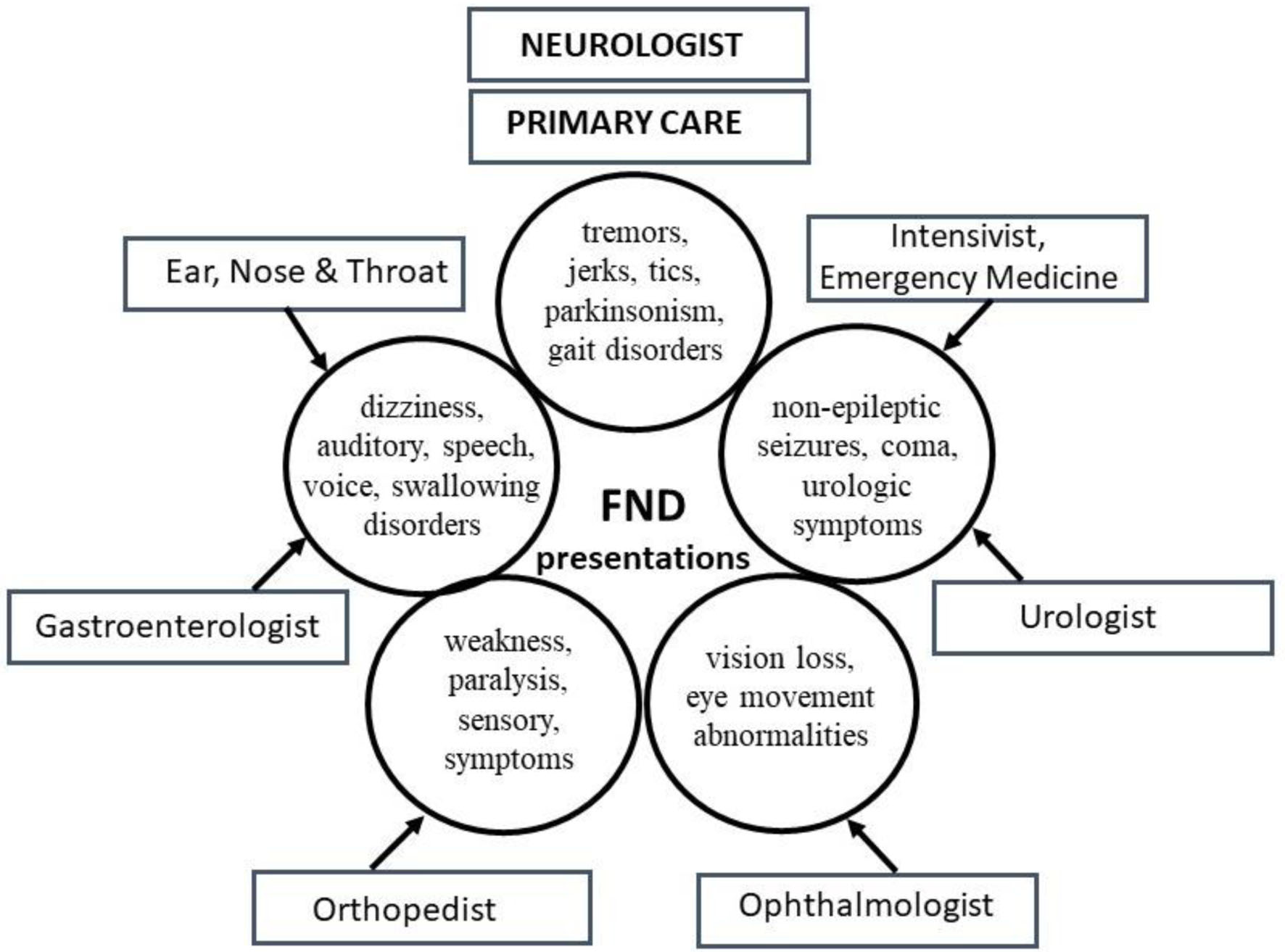 Click for large image | Figure 1. Common functional neurological disorder (FND) presentations and specialists involved in their care. Multiple symptoms grouped together depict the complex FND presentations encountered by various physicians simultaneously. Neurologists and primary care physicians (PCPs) evaluate most symptoms. |
An expanding umbrella of FD is being increasingly recognized, under which multiple symptoms and conditions affecting various organs and systems are identified. For years, FND experts have understood that some of the commonest extra-neurological presentations of FD include fibromyalgia, chronic fatigue syndrome, and irritable bowel syndrome [10] (Fig. 2). Recent evidence suggests shared risk factors and pathophysiological mechanisms underlying many of these conditions [11].
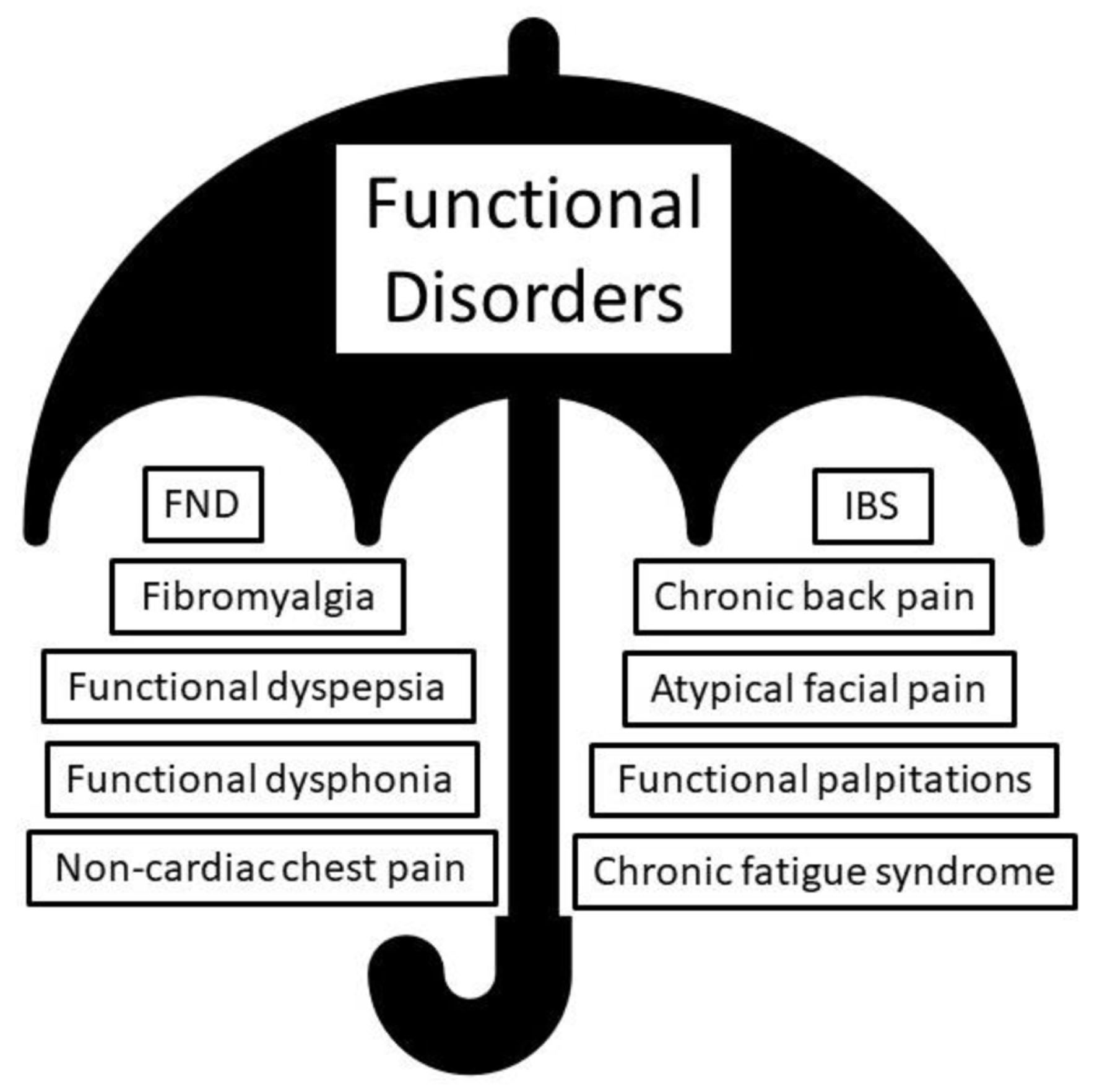 Click for large image | Figure 2. The umbrella of functional disorder (FD), containing functional neurological disorder (FND) and other extra-neurological functional diagnosis. |
Common dysfunctional cortical and subcortical regions and pathways, involving brain structures mediating emotional, autonomic, and sensorimotor (somatic) functions, such as the thalamocortical pathways, the somatosensory cortex, the insula, and the amygdala, are shared between several FDs, such as FND, pain disorders [12] and migraine [13, 14]. Studies report overlapping dysfunction across four major FND phenotypes: movement disorder, seizures, cognitive and dizziness, without significant pathophysiological differences, for example defective sensory processing, limbic-motor, cognitive-emotional networks, attention and interoception [11, 15] have been noted across subtypes. Patients with functional motor disorders often reveal similar clinical manifestations, such as depression, anxiety, pain, fatigue, and sensory symptoms [16, 17]. This evidence favors the presence of a complex and large, shared, series of pathophysiological mechanisms interlinking various FND subtypes.
Despite this pathophysiological overlap, in clinical practice, the FND diagnosis is quite reductionist, and typically assigned according to the predominant symptomatology or phenotype observed during the encounter, such as FND with mixed symptoms, abnormal movements, or weakness (Fig. 3). This approach may lead providers to infer that each FND subtype represents a distinctive etiology and pathophysiology, characterized only by the clinically manifested defective neural network. For example, while managing a patient with functional motor disorder, the physician recognizes the dysfunctional motor pathways, but may overlook the abnormal limbic, sensory, and attentional processes often present and in need of treatment. The noteworthy overlap in pathophysiology across FND subtypes should be considered when designing universal management protocols. Equally, individualized management contemplating all the functional manifestations of each individual’s presentations is needed.
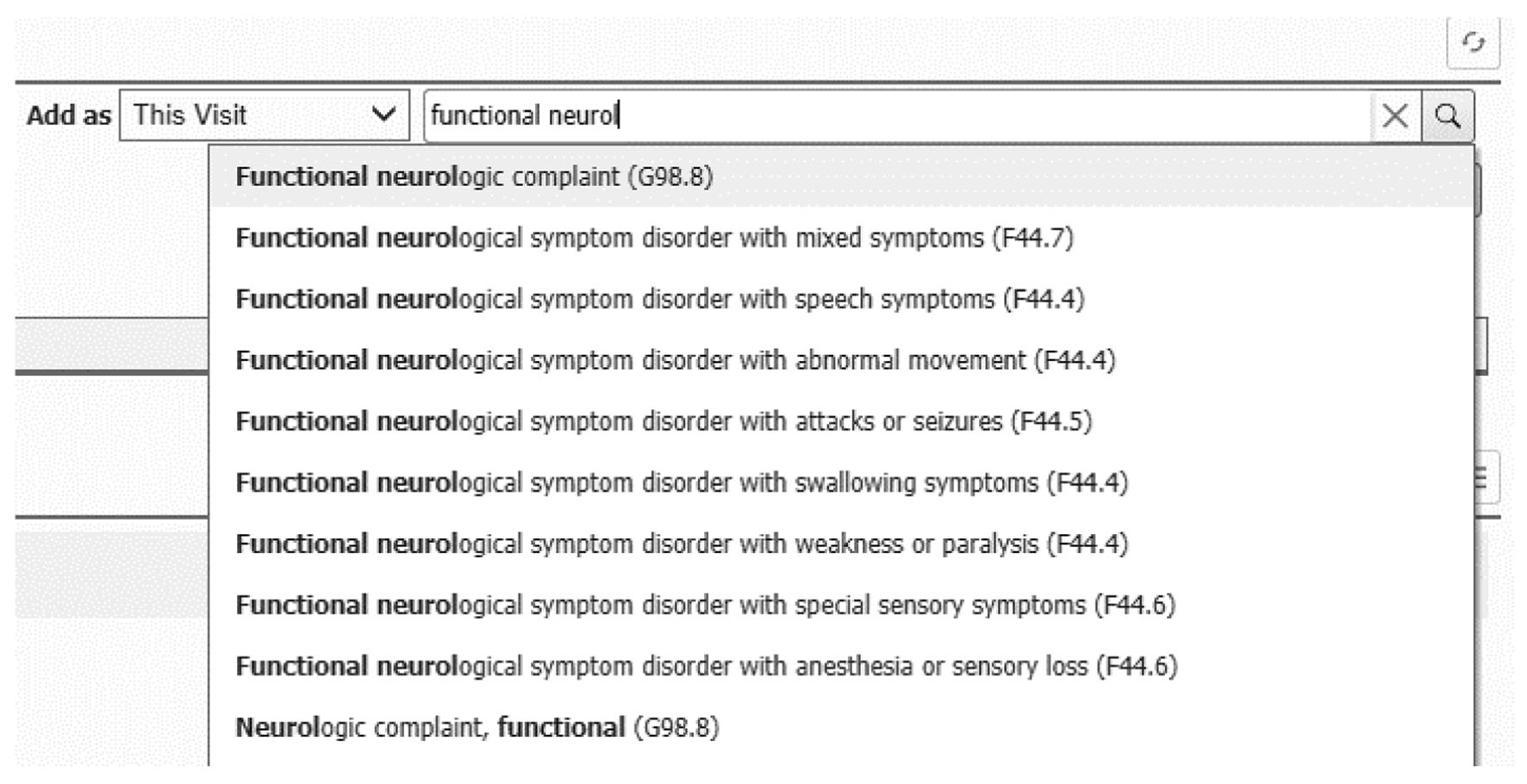 Click for large image | Figure 3. Electronic healthcare record screenshot demonstrating functional neurological disorder (FND) sub-diagnostic categories. |
FND patients frequently have multiple psychological and functional physical symptoms, from various organs and systems simultaneously [18-21], such as anxiety, dissociation, fatigue, cognitive, sensorimotor (somatic) disturbances, more frequently than patients with organic neurological problems [22, 23], often associated with lower quality of life (QOL) [24-26], and poor outcomes [27, 28]. Avoiding mind-body dualism [29] and addressing psychological factors even when they are not easily apparent [8], have been emphasized. However, there are no guidelines about the extent and scope of the psychological assessment to orient the various specialists involved in FD care, including the primary care physician (PCP), who is often responsible for initial management.
This briefly analyzed classification consideration about FD and FND identifies the following remarks (Table 1).
 Click to view | Table 1. Factors Concerning FND and FD Classification and Management |
Multidisciplinary collaboration in classification and management
Given the many symptoms individuals with FD present, it would be advantageous for neurologists to collaborate with other specialists, including rheumatologists, pain management, gastroenterologists, as well as members of the DSM workgroup, to create a more accurate and comprehensive mind-body classification system, clarifying that what is being increasingly recognized is an umbrella of FD. Individuals with FD present with conditions such as fibromyalgia, chronic fatigue syndrome, functional dyspepsia, irritable bowel syndrome (IBS), functional palpitations, and non-cardiac chest pain [10]. In alignment with this, experts in medical nomenclature and classification should consider updating the terminology permeating FD. For instance, 1) a grouping of functional rheumatological disorders (FRDs), including conditions such as fibromyalgia, and chronic fatigue syndrome, 2) functional gastrointestinal disorders (FGIDs) embracing functional dyspepsia and IBS, and 3) functional cardiovascular disease (FCVD), comprising functional palpitations and non-cardiac chest pain. This updated classification system, grouping functional diagnostic entities according to organs and systems, will allow for a more thorough study of the dysfunctional processes taking place in the sensory-motor structures of each system in connection with the abnormal central nervous system networks. Such an approach would strengthen collaboration between neurologists and clinicians from different specialists, allowing them to obtain a deeper understanding of the pathophysiological mechanisms affecting the organs and systems, ultimately improving management algorithm and patient outcomes.
From such a functional understanding, a comprehensive management protocol capable of targeting the shared pathophysiological mechanisms across FD will be more easily developed.
| Epidemiological and Social Trends | ▴Top |
Older and modern risk factors
Risk factors for FND follow a biopsychosocial model, and include adverse physical, emotional, mental, and environmental circumstances that can occur at early ages (Fig. 4) [30-34]. Although this review focuses on adult FND, it is necessary to provide key data on pediatric FND to demonstrate the relationship between life stages and the childhood-adulthood functional continuum, as characteristics of FND in childhood will inform adult management and disease prevention.
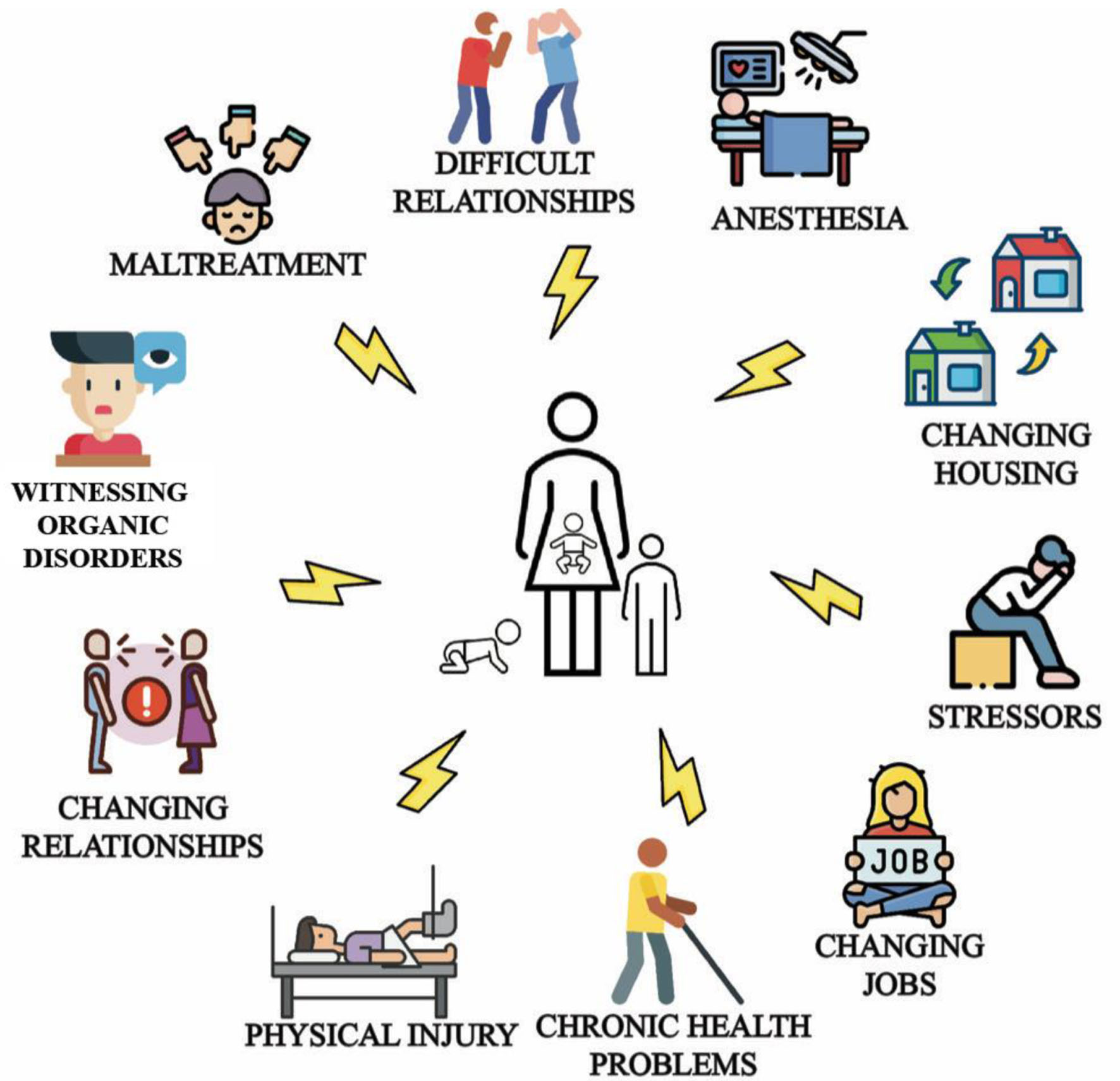 Click for large image | Figure 4. Common triggers and risk factors for pediatric and adult functional neurological disorder (FND) that characterize the biopsychosocial model: emotional antecedents, work problems, accidents, family difficulties [30], change in relationship, housing, employment status [31-33], and witnessing functional or organic movement disorders [34]. |
Adult FND patients tend to have a history of traumatic childhood experiences [35, 36], such as sexual [35], physical, or emotional abuse [31, 37-39]. Most adverse life events described in the adult FND population are rooted in childhood [40].
The most studied pediatric FND subpopulation, functional seizures (FS), have a high frequency of preexisting and newly diagnosed psychiatric disorders [41], such as emotional, adjustment, attachment disorders and post-traumatic stress disorder (PTSD) [42]. Adults with FND also have rates of depression between 20% and 40% [43, 44], anxiety approximately 38% [45], and Axis I emotional disorder between 66% and 75% [46]. Both children and adults have high rates of psychological manifestations.
Increasing incidence
The worldwide distribution of FND has been rapidly increasing. The incidence rate of FND between 1976 and 2010 was 4 to 12 per 100,000 patients per year [21, 47-49] and the prevalence was 50 per 100,000 population [50]. In 2010, FND was found to be the second most common reason for new outpatient neurological evaluation [51]. In 2020, FND was recognized as frequently co-occurring with migraine, another common neurological disorder [52]. In 2021, FND started to be diagnosed after COVID-19 vaccination [53, 54], despite it lacking neurotoxic effects, leading to vaccination hesitancy [54-56]. In 2021, a study suggested that TikTok and social media could be contributing to the spread of FND [57].
FND is also increasing in children and adolescents. In a Danish nationwide study, the overall incidence rate increased from 2.4 per 100,000 population between 1996 and 2014, the full study period, to 7.4 per 100,000 between 2005 and 2014, with its peak in 2014 [58]. Up to 23% of children with FND retain the diagnosis or demonstrate recurrence during adulthood [59].
Little research has been conducted to understand the rising rates of FND. Factors contributing to this surge likely include an increased interest in FND, scientific advancements in the field, the educational work performed by the Functional Neurological Disorder Society, and the improvement and dissemination of diagnostic criteria, all likely promoting FND recognition. The biopsychosocial model stimulates reflections about modern society’s stressors influencing FND’s incidence, and the role of the educational system and families in teaching individuals to effectively deal with adverse circumstances. Regardless of the factors, FND is a global problem, and the field is in need of more epidemiological research.
Mortality
Patients with FS and functional epilepsy (FE) have an increased risk of death [60-62]. One study found that patients with FE younger than 30 years old have up to eight-fold higher risk of death compared to the general population, and that 20% of those younger than 50 years old committed suicide [60]. Suicide is also high in patients with functional motor disorder [62].
Having a psychiatric comorbid condition has been reported to be associated with higher mortality rates [63, 64], and 49% to 100% of patients with FS and FE have a concomitant psychiatric diagnosis [65, 66]. It is reasonable to assume that psychological wellbeing influences FND clinical outcomes.
The brain networks involved in human psychology start developing during the prenatal period, with vital moments during childhood and adolescence; addressing how those years impacted the nervous system of those with FND, must be considered in all management protocols.
Financial and social impacts
Research on FS, one of the most studied subtypes of FND, indicates considerable financial strain on healthcare systems. In Australia, a person with FS utilizes healthcare services at a median of $26,468 Australian dollars [67]. In the USA, the lifetime cost is around $100,000, an expenditure similar to treating intractable epilepsy [68].
The FND population incurs high inpatient and outpatient healthcare expenditures, due to frequent primary care, specialty, emergency room evaluations, and hospital admissions [69]. FND patients frequently change PCPs and undergo multiple referrals [70], with the rarely discussed emotional, mental, and financial consequences triggered by such frequent switches in physician-patient relationships. The field is in need of studies seeking to identify preventive measures for such common phenomenon.
Adults with FS and their partners also generate significant social impact due to socioeconomic deprivation and lower employment rate [71]. In Denmark, direct and indirect annual costs of sick pay, disability pension, and home care services approximate €33,697 for patients and €15,121 for their partners [72]. Caregivers experience social stigma [73], psychosocial adversities, and reduced QOL [74]. The literature describing the socioeconomic impact of FND consistently finds a high financial cost [62, 72, 75-77].
FND has triggered BBC, CNN, and other media platforms’ interest. Thirteen news stories were shared between 2012 and 2017 portraying patients with complex symptoms being misdiagnosed or presented as medical mysteries [78], advertising the deficits that still exist in FND management.
FND prevention
As previously noted, there is little data to guide the prevention of FND. As primary prevention aims to reduce disease incidence, the absence of such information delays our ability to stop, or at least slow, the increasing incidence and prevalence of FND.
A review of FND risk factors, incidence, financial, social impacts, and mortality, suggests the need to consider many factors to optimize FND management (Table 2).
 Click to view | Table 2. Considerations Regarding FND Epidemiology and Socioeconomic Burden |
FND management focused on biopsychosocial-spiritual assessment
Considering the biopsychosocial triggers, risk factors, and the childhood-adulthood continuum, a comprehensive FND assessment and management protocol would benefit from adopting a biopsychosocial-spiritual model of disease [79, 80], with a biographically focused approach considering how the patient’s experiences since the prenatal period and across time, have contributed to the patient’s current presentation.
Given the rapidly increasing incidence of FND and its presentation to multiple specialties, we are of the opinion that a biographically focused biopsychosocial-spiritual model is needed across medical practices. Such approach can bring to the patient’s awareness the significance of past adverse events, enhancing the patients and families’ understanding of FND, promoting enrollment and adherence to therapy. For example, systematic discussions with adult FND patients about how their past experiences have impacted them physically and psychologically, could increase their capacity to overcome challenges, build resilience, influencing clinical outcomes and informing preventive tools.
In sum, guideline recommendations addressing the providers’ approach to the patient-physician relationship should consider the biographical and epidemiological data pertaining to the FND population, characterized by adverse events and traumatic experiences. Concepts derived from attunement, attachment theories, defense mechanisms, the functioning of the limbic system, social, and cultural understanding, should be considered when creating such guidelines. Across time, partnerships between the medical community, media outlets, social media platforms, and policymakers could serve as effective means to transfer knowledge to members of the public.
| Outcomes and Prognosis | ▴Top |
Therapeutic modalities for FND
A review of 24 studies performed between 1940 and 2013 involving functional tremor, dystonia, parkinsonism, weakness and FND with mixed symptoms, described complete remission in only 20% of the patients, with 40% staying the same or becoming worse over a 7.4 years follow-up period [27]. Outcome studies performed between 1957 and 2006 in patients with functional tremor also reported poor prognosis, with 44% to 90% of patients doing the same or worse upon follow-up [81-84]. Other studies between 2003 and 2010 find that most patients with FND with mixed symptoms have a poor outcome [85, 86].
A similar pattern of outcomes is observed across the world. A study conducted in Sweden in the late 1990s found that 43% to 89% of the patients with functional motor symptoms were out of work [87]. Studies conducted in Canada and the United Kingdom the early 2000s found that 38% to 58% of patients develop other functional symptoms at follow-up, especially weakness [45, 88]. By 2011, less than 30% to 40% of the patients with FS demonstrated remission [89]. Given the disappointing outcomes and prognosis, neurologists have focused on improving the treatments provided to the FND population, including physical therapy (PT) and psychotherapy.
First-line body-oriented modality: PT
PT is an integral part of the treatment of many FND subtypes. PT is used to retrain movement, redirect attention, positively influence illness beliefs [90], trigger plastic adaptation by repetition and task-oriented exercises [91]. In this manner, out of all psychotherapeutic modalities, PT often incorporates principles from cognitive behavioral therapy (CBT). Symptom retraining appears more effective when directed to the underlying mechanisms driving the functional symptoms [92], suggesting the importance of pathophysiology even during PT sessions.
Various PT techniques have demonstrated benefits, such as movement retraining for functional motor symptoms [93], and walking for functional movement disorder [94]. Bicycling, canoeing, and indoor climbing [95], studied in functional gait disorder, resulted in improvements lasting greater than 12 months. Group exercise showed benefits in those with mild to moderate functional symptoms [94]. A review of 33 studies [96] utilizing interventions lasting from 5 to 100 days, revealed significant improvement in over 48% of patients, although complete symptom resolution was low.
Physiotherapy for FND continues to be investigated. A 2016 randomized feasibility study of PT for FND with motor symptoms [97] followed 29 patients who received eight 45 - 90 min therapy sessions over 5 days, mainly utilizing education, movement retraining, attention redirection, investigating triggering events, comorbidities, psychological factors, features of self-focused attention, and unhelpful reinforcements. The program promoted daily reflections and the development of a strategic and individualized symptom formulation and management plan. At 6 months, 72% of the individuals receiving the interventions rated their functional motor symptoms improved or much improved in the Clinical Global Impression Scale. Randomized controlled trials (RCTs) are needed to determine if outcome can be further improved via utilizing additional techniques and modalities capable of targeting the patient’s underlying pathophysiological mechanisms.
Comprehensive physiotherapy consensus recommendations for functional speech, swallowing [98] and occupational therapy [99] have been published. However, it is unclear how applicable these are to daily clinical assessment and management, outside a physiotherapy session. Most of the assessment and therapeutic interventions recommended are CBT-derived.
First-line psychotherapy: CBT
CBT is a top-down psychotherapeutic modality that works under the premise that a person’s thoughts and beliefs affect feelings and behaviors. During CBT sessions, patients are assisted in the identification of cognitive misrepresentations and dysfunctional beliefs, to disrupt coupled cognitive, emotional and behavioral responses to stressors [100].
The evidence supporting the use of CBT in FND goes back to the last century. Kroenke and colleagues found CBT treatment for somatoform disorders effective in 11 out of 13 studies performed between 1966 and 2006 [101]. In 2004, a small open-label study utilizing a 12-session CBT intervention suggested that CBT could aid in the reduction of FS frequency [102]. In 2014, two pilot RCTs using CBT with or without standardized medical care (SMC) were conducted. One suggested efficacy of CBT alone in patients with FS, reducing seizure frequency during the treatment period in 51.4% of patients [103]. This study, however, was insufficiently powered to allow comparisons between treatment arms. The other study, through an intention-to-treat analysis, demonstrated a greater reduction of seizure frequency in the CBT plus SMC. However, 6 months after the end of treatment, the difference between the groups was no longer significant [104].
Trauma-based CBT has demonstrated benefits in the FND population with associated PTSD [105]. A study using CBT or acceptance and commitment therapy achieved and maintained improvement in FS frequency and healthcare costs at 2 years [106]. CBT-informed psychotherapy (CBT-ip) for functional tremor resulted in a significant reduction (78.7±33.8%) on the psychogenic movement disorders rating scale 12 weeks after completing CBT [107].
The first RCT involving CBT for FS patients was conducted in 2015, assessing the effect of adding CBT to SMC and psychiatric care in 368 patients [108]. The Cognitive Behavioral Therapy vs. Standardized Medical Care for Adults with Dissociative Non-Epileptic Seizures (CODES) trial included a care pathway involving neurology and psychiatry. The CBT interventions taught distraction and refocusing techniques to interrupt seizures, addressed avoidance behaviors, unhelpful beliefs, trauma processing, and stress management. The primary outcome measure, reduction in FS frequency in the previous 4 weeks, assessed at 12 months, was not met. There was no significant difference in FS frequency between the groups, which demonstrated, in the previous 4 weeks, a median of four seizures in the CBT plus SMC group, and seven seizures the SMC group. The authors proposed various explanations for the findings, such as the limited trauma-focused interventions in a population with significant traumatic history, the possibility that only certain patients might be appropriate for CBT-based treatment, whereas others may benefit from other psychotherapeutic modalities. More recently, the authors suggested that perhaps the impact, or lack thereof, of specific CBT interventions, in certain pathophysiological networks, such as physiological, emotional, spiritual, and social aspects permeating FND production and maintenance could have played a role in the failure to significantly reduce FS frequency [108, 109].
Similar deficits in treatment are found in the clinical trial results in pediatric populations, with low rates of early and sustained remission, high rates of symptom persistence [110] and recurrence, even after multidisciplinary treatment [111, 112]. The Retraining and Control Therapy (ReACT) trial, an RCT utilizing CBT principles to target sense of control and symptom expectations [113], demonstrated symptom recurrence in two (18%) of the participants after 60 days of treatment completion. Mental health services at 1 month were associated with remission at 12 months, again suggesting the importance of addressing psychological health early. Psychosocial stressors have also been associated with unfavorable outcomes in pediatric populations [114], suggesting the importance of a prompt focus on stress management.
Due to the foundational principles of CBT, its most frequently utilized techniques focus on the cognitive and behavioral mechanisms of FND production and maintenance [115]. CBT is characterized by fewer interventions in the emotional, biological, autonomic, and sensorimotor (somatic) domains of the human nervous system (Fig. 5). A focus on these latter areas may be required to address the unconscious dynamics associated with FND.
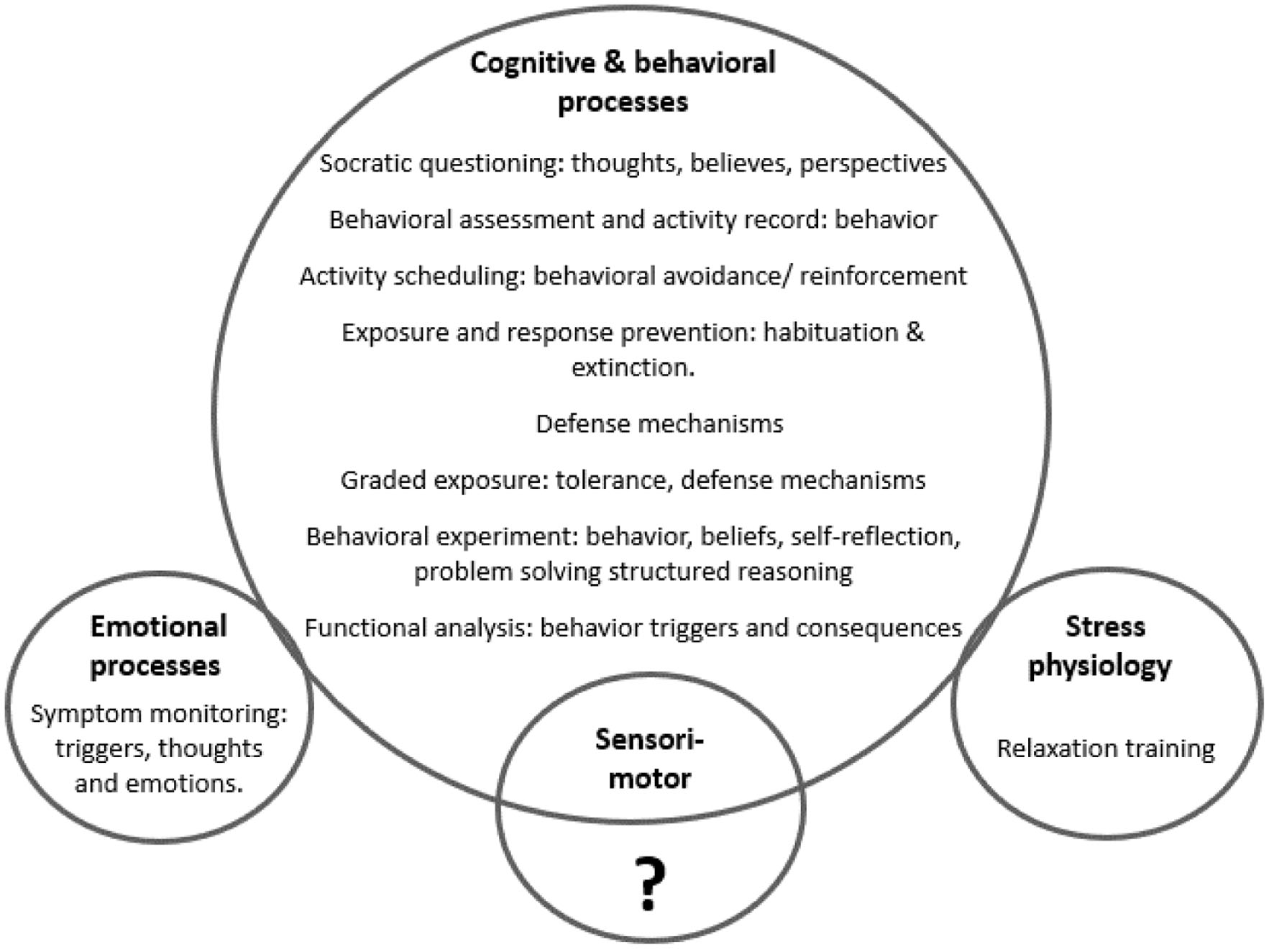 Click for large image | Figure 5. Frequently used cognitive behavioral therapy (CBT) techniques, demonstrating a greater influence in cognitive and behavioral processes, with unclear impact on the sensorimotor (somatic) system, and less impact in the biological (stress physiology) and emotional domains of the nervous system. |
Evidence for other therapeutic approaches
Carlson and colleagues conducted a meta-analysis with data extracted from 13 studies involving 228 participants with FS who received various psychotherapeutic modalities, including psychodynamic therapy, paradoxical intention therapy, mindfulness, psychoeducation, and eclectic interventions [116]. Results showed that 47% of patients became seizure-free upon completion of any psychological intervention, suggesting that various modalities are helpful in FND. The study did not compare or demonstrate superiority of one intervention over the other.
Principles from psychodynamic therapy have been effectively utilized to reduce functional movement disorder [117] and other types of FND [118-120]. It has been suggested that interventions from psychodynamic therapy may effectively address vulnerability factors involved in FE [121]. The need for psychotherapeutic modalities that target emotional and attachment dynamics is increasingly recognized [121-123].
Two prospective uncontrolled studies of mindfulness-based psychotherapeutic approaches conducted in FS patients to improve awareness and acceptance of internal states demonstrated improvement in FS intensity and worry [124, 125]. In one of the studies, some outcomes were maintained 6 months post-treatment [124].
Other treatment modalities showing some evidence for children and adults with FS are education [126], biofeedback [127-129], family therapy [130], group therapy [120], multidisciplinary care [114, 131] and inpatient treatment programs [127]. Recommendations for treating adults and children with FS specify that mood and trauma should be targeted [103, 127].
Multidisciplinary programs
Multidisciplinary or multimodal care for FND involving family medicine, neurological, psychiatric, psychological care, and if needed physiotherapy, are being used in clinical practice [132-135], although they are not widely available, and often require patients to access care at far away hospitals and academic centers. The first published proposal of a multidisciplinary clinical care pathway for FND was performed in children with FS. This care pathway utilized information from a retrospective study that characterized this population from epidemiological and clinical perspectives, paying attention to psychiatric comorbidities and psychological assessment. The authors considered the interventions used, mainly education and CBT, and their outcomes [133]. This multidisciplinary clinical care pathway was then prospectively validated [136] by providing specialized neurological and psychological care to 43 children with suspected FS over a 5-year period. Length of treatment went from 1 to 24 months, and CBT was utilized for most patients (n = 31, 72%). Patient education, group therapy, family therapy, biofeedback, and trauma-based CBT were also used.
As part of the pathway’s protocol, psychophysiological assessment, biofeedback training and strategies to avoid hyperventilation were implemented following the notions that emotional and autonomic abnormalities underlie the pathophysiology of FE. Some patients were referred to somatization clinics, inpatient or intensive outpatient mental health, and/or rehabilitation programs. Continued outpatient follow-up was encouraged. Dissociation, characterized by depersonalization, and derealization was managed according to published recommendations [137]. Retrospective chart review demonstrated that standardized combined care led to self-reported full or partial remission at discharge in 27 (63%) and 12 (28%) children, respectively. The only statistically significant factors associated with incomplete response were the presence of delayed diagnosis, and duration of FE greater than 12 months. The authors recognized as a limitation of the study the absence of random assignment to treatment.
Other models for clinical care pathway formation in the adult FS population have been reported [138], and a multidisciplinary approach to FS treatment is recommended [132]. An expert consensus board review determined that FS management for both children and adults should be multidisciplinary and should include CBT [139]. In functional movement disorder, 1-week multidisciplinary rehabilitation program resulted in an 86.7% symptom improvement upon treatment completion, which decreased to 69.2% at 6-month follow-up [140].
Multiple interpretations can be derived from these data. Complete and sustained remission is a goal that is not yet consistently attained, even with multimodal or multidisciplinary care. This is perhaps due to the predominant utilization of CBT modalities, the lack of easily accessible services, the high number of providers involved in care, and the need to utilize outside referrals, with its financial, and logistic implications. Psychiatric, psychological, psychophysiological assessment, somatization management, and education appear valuable, supporting the need to comprehensively address all systems involved in the production and maintenance of FND.
Current outcomes and unanswered questions
Across studies examining the effectiveness of different treatments for FND - PT, CBT, and multidisciplinary approaches - complete symptomatic remissions in FND are rare, with many patients remaining refractory to the currently available therapies [141]. Unfavorable prognosis, with many patients having persistent symptoms after years of follow-up, is common [27]. A 14-year follow-up of patients with functional limb weakness found ongoing symptoms in most, with greater than expected mortality [142]. Inpatient programs show that, whereas improvements in functional motor symptoms and QOL are probable, disabled patients often do not return to work [140, 143, 144]. The prognosis of FND remains poor, with disability persisting or worsening over time, and with many patients experiencing severe symptoms despite treatment [145].
Regarding psychotherapy duration and healthcare delivery methods, one study demonstrated greater efficacy of CBT-ip for FS, if at least seven sessions were administered over more than 3 months, versus within 3 months [146], suggesting that prolonged engagement in treatment, or a personalized treatment duration, may be more beneficial than shorter standardized therapeutic protocols. The ideal psychotherapy duration has not been clearly determined by RCTs.
Telehealth has been proposed as an appropriate way of delivering psychotherapy [147] and PT [148]. Telehealth could facilitate the care of patients with mobility and financial difficulties, although the efficacy of this approach has not been sufficiently studied. Research in this area is of interest.
There is also a need for better outcome measures for FND. None of the current well-validated outcome evaluations consider all the core features of FND, such as symptom heterogeneity, variability, illness beliefs, social support, comorbid psychological and medical problems, and the role of the clinician collecting a great deal of subjective data such as pain or fatigue [149, 150]. In addition, the percentage of patients who progress to chronicity despite utilizing the best possible treatments, remains unidentified. Therapeutic approaches should be scrutinized against FND pathophysiology to determine if the most favorable therapeutic modalities are currently being delivered.
Key findings from the analysis of outcomes, prognosis, and FND therapeutic modalities are summarized in Table 3.
 Click to view | Table 3. Findings From FND Treatment Studies |
| Therapy and Management for FND | ▴Top |
Pathophysiological mechanisms of FND
The pathophysiological mechanisms of FND may include the following: 1) Childhood trauma and epigenetics [151]; 2) Psychological model and the limbic system [115]; 3) Defense mechanisms [152]; 4) Cognitive and learning theories [151]; 5) Hypnosis model [153]; and 6) Stress and neurobiological theories [153].
Summarizing in a single model, all the pathophysiological evidence described in the above theories, can deepen our understanding of the dysfunctional processes taking place within the nervous system, and affecting the physical body, while avoiding mind-body dualism. All the functional networks that play a role in FND origin and maintenance are biologically interlinked. Thus, theoretically assembling them offers a foundation to develop an integrative treatment algorithm.
The pathophysiological processes affected in the above etiological models can be grouped into four functional networks (Table 4) [116, 151-154]: 1) Neo-cortex; 2) Limbic; 3) Autonomic nervous system (ANS); and 4) Sensorimotor (somatic) processes extending from the nervous system to the interconnected physical regions.
 Click to view | Table 4. Summary of FND Pathophysiological Theories With Supporting Dysfunctional Networks Demonstrating Near-Constant Impairment of Multiple Processes Within Four Major Functional Areas: Limbic System, Neo-Cortex, ANS, and Somatic (Sensorimotor) |
The involvement of these four functional regions, limbic system, neo-cortex, ANS, sensorimotor processes, in the pathophysiology of FND has been demonstrated in the studies cited in the previous sections. For example, when discussing therapies targeting cognition and behavior such as CBT, the presence of somatic complaints, the involvement of the limbic system manifested through associated mood disorders and psychological co-morbidities, and the ANS influenced by traumatic and stressful risk factors are all of note.
Common pathophysiological processes across various FND subtypes have been demonstrated in clinical trials [155, 156], and the need for a transdiagnostic approach capable of therapeutically targeting shared dysfunctional cognitive, emotional, autonomic, behavioral, and social dynamics, has been proposed [157, 158].
The inter-relatedness between these four functional networks and the importance of comprehensively and systematically targeting them all is manifested, for example, when hyperarousal (autonomic), anxiety (mood, emotional), readiness for change (cognitive) and symptom count (somatic) were noted in functional movement disorder patients during triage to therapy [159]. Other studies have found that hypo/hypervigilant states, sleep disturbance, motivation, mood disturbances, anxiety disorders, alexithymia, trauma, chronic fatigue, and chronic pain [160, 161], representing autonomic, cognitive, limbic, and sensorimotor processes respectively, were involved in the neurocognitive dysfunction of patients with FE.
Multiple studies have demonstrated abnormalities permeating the limbic-motor systems [162, 163], limbic-sensory processes [164], and cognitive-unconscious dynamics [165], some of them in relationship to the motor system [166-168], others associated to the limbic system [169], the sensorimotor pathways [170, 171] and the social network [172]. A large body of evidence speaks about the involvement of the ANS [169, 170, 173-176].
The frequent dysfunction in these four networks: limbic, cognitive, autonomic, and sensorimotor (somatic), has not been systematically assembled into educational and management models.
We introduce an example of how assembling all pathophysiological theories and mechanisms described to cause FND in a single model would look: the integrative FND pathophysiological model (Fig. 6). Our example contains four outer boxes summarizing the theories explaining FND production, in both adult and pediatric populations: 1) Left upper box: During the prenatal period, stress in mothers can affect fetal brain development and later functioning as the mother and fetus share the hypothalamic-pituitary-adrenal (HPA) axis. Throughout this period, stress and trauma can alter a person’s genes and their expression, leading to dysfunction in certain nervous system networks. The type of attachment or emotional bond between individuals can impact the operations of nervous system pathways, especially the limbic (emotional) system. 2) Left lower box: Multiple mental, emotional, physical, and social adverse and traumatic events can impact the performance of neuronal networks. 3) Right upper box: During a person’s life, cognitive, learning, conditioning factors, beliefs, and suggestions can determine the brain’s software. “Rogue schemes” and “priors” refer to mental representations and predictions that influence the nervous system’s processes. Therefore, numerous situations can result in FND. Fortunately, we understand what brain pathways and networks can become dysfunctional due to the above situations, even when the specific etiology seems unclear (right lower box).
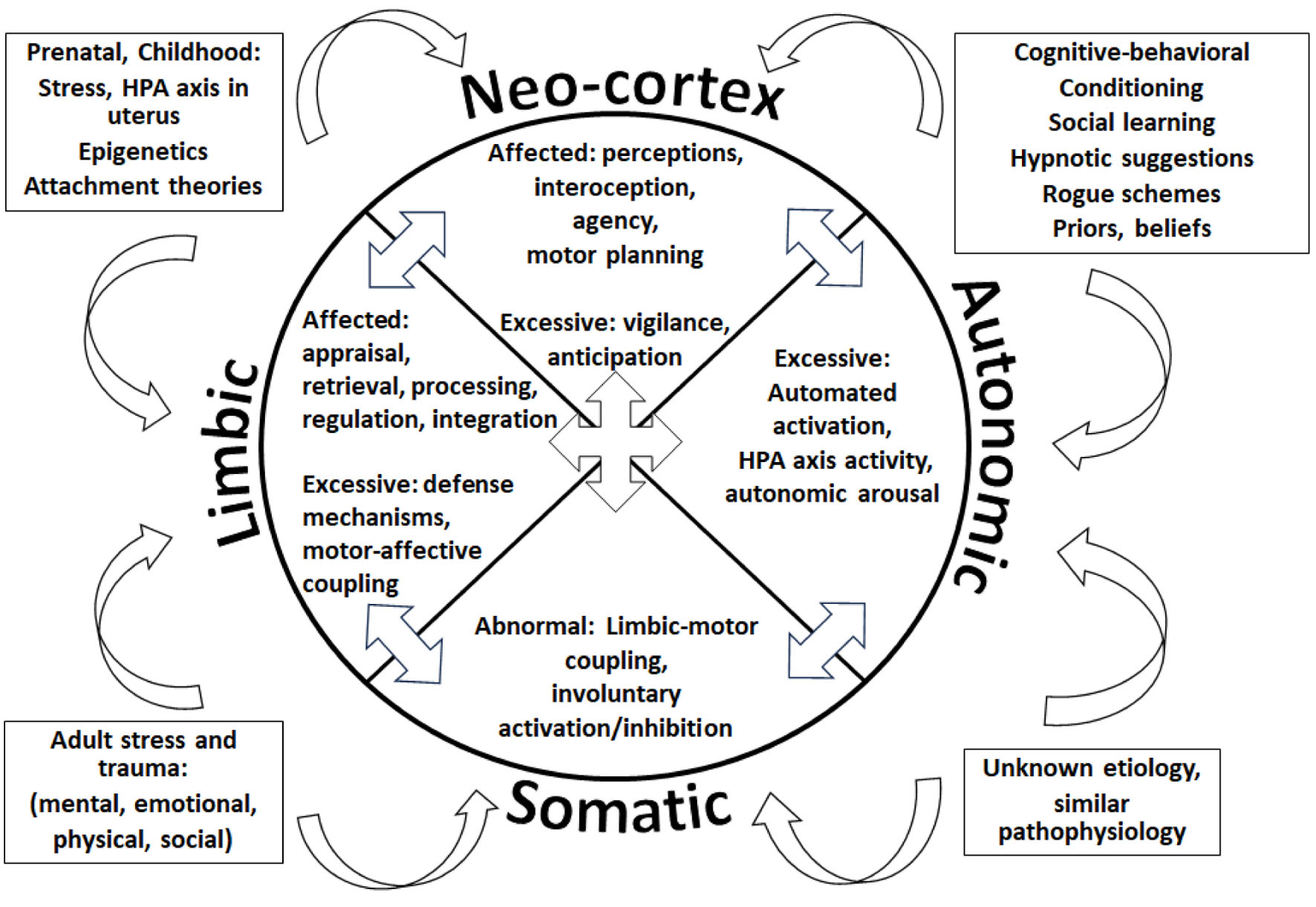 Click for large image | Figure 6. The integrative functional neurological disorder (FND) pathophysiological model representing dysfunctional processes in the cognitive neo-cortex, the limbic system, the autonomic nervous system (ANS), and the sensorimotor (somatic) processes. |
All the etiological processes represented inside the boxes, independently or in combination, can influence the nervous system, ultimately affecting one or more of its four large functional regions, denoted by each quadrant of the center pie: the cognitive neo-cortex, the limbic system, the ANS, and the sensorimotor (somatic) processes. Inside each quadrant or functional region, some of the dysfunctional processes described in the literature are represented: 1) In the limbic system, a person’s emotional appraisal [168, 177], retrieval [166], processing, regulation [167], and integration [165] could be affected. Excessive defense mechanisms may be present [165], and sometimes motor functions can be abnormally linked to emotional processes [164]. 2) In the neo-cortex, the individual’s perceptions [178], interoception [179], agency [180], motor planning [181] could be defective, while vigilance [182], and anticipation [183] may be excessive. 3) The patient’s somatic (sensorimotor) networks could demonstrate abnormally tight connections to emotional processes (limbic-motor coupling) [163, 181], sometimes getting automatically activated or inhibited [170, 184]. 4) The person’s ANS may show excessive activation [174], HPA axis activity [185, 186], and autonomic arousal [174].
The arrows located between the pies indicate that the abnormal functions inside a quadrant could affect the operations of other functional regions.
The reader must consider the separating lines between functional quadrants and the grouping of the procedures inside each quadrant, as purely symbolic. Human experience is dependent on this intricate web of functional operations, working interdependently, and largely subconsciously.
The distribution of a thoroughly designed integrative pathophysiological model could help healthcare professionals understand the processes originating and maintaining FND, with its many possible presentations. It could also facilitate the recognition of the many emotional, autonomic, somatic, and cognitive manifestations of FND, helping to validate the patient’s experience, preventing their dismissal, discharge, and transfers of care, leading to better management, proper allocation of services, and improved healthcare utilization. An integrative pathophysiological model could be utilized at first encounter with all suspected and confirmed FD patients, improving a patients’ understanding of their condition and their engagement in therapies. Such a model could also be used to teach trainees and healthcare professionals about FND production and maintenance.
An integrative pathophysiological model could prompt physicians to investigate with patients, in a biographically and biopsychosocial-spiritual manner, how their risk factors, represented by the outer boxes, could have given origin to dysfunctional limbic, autonomic, sensorimotor (somatic), and cognitive processes.
Of course, the integrative FND pathophysiological model presented here is just an example, describing some of the pathophysiological processes described in the literature, and created to demonstrate the usefulness of an integrative approach. An in-depth literature review designed to identify, analyze, and describe all pathophysiological processes that have been proposed in the literature to affect the limbic system, neo-cortex, ANS and sensorimotor networks - while considering their interrelatedness, clinical manifestations, relevance, assessment, and optimal targeting modalities - should be undertaken.
Multi-network management algorithm for FND
A multi-network treatment algorithm is founded in an all-inclusive pathophysiological model and targets all the dysfunctional processes within the limbic, autonomic, sensorimotor (somatic) and cognitive networks, regardless of the patient’s identified etiology or lack of thereof, as some patients describe not having been exposed to the most common risk factors [31].
All functional processes known to be affected in the FND population should be assessed in all patients, and therapeutically targeted when necessary. Such a thorough approach will be similar to giving broad-spectrum antibiotic coverage for an infection caused by a yet unknown pathogen; the chance of a cure despite an unidentified cause is much higher. This strategy is particularly necessary now that the availability of diagnostic tools such as functional neuroimaging, capable of identifying dysfunctional networks, is mostly restricted to academic centers. And, even if the widespread availability of imaging technology capable of detecting dysfunctional networks was to occur, it is unlikely that such a diagnostic instrument would identify the subtle dysfunctional processes (e.g., emotional dysregulation, hypervigilance, lack of interoception, etc.) that are fairly easily found via a comprehensive clinical interview. An integrative, pathophysiologically focused assessment and management tool can be made available to rural areas, private practices, and smaller community hospitals.
Although recent reviews advocate for the creation of individualized treatment protocols [11], the methodology to deliver such care is not clear. Current FND treatments vary tremendously, from focusing on the psychophysiological mechanisms behind functional seizures to, in functional motor disorders, noticing the differences between voluntary and automatic movements, using focusing techniques on some cases, while in others, utilizing distraction. The heterogeneity in FND clinical manifestations, severity of illness, the individual’s biology, life experiences, socio-spiritual dynamics, and psychological well-being may appear too varied to standardize treatment. FND management must be simplified and systematized, by focusing on the individual’s pathophysiological manifestations, which are limited in number, well described in the literature, and often overlap across the umbrella of FD. From a well-known pathophysiological foundation, patient and clinician can become active participants in the design of a path towards functional rehabilitation [187].
To our mind, individualized treatments should consist of having, and delivering to patients, predetermined tools capable of addressing the dysfunctional processes that could be taking place inside each of the four described networks. The pathophysiological processes can be detected through physical examination and then targeted through clinical intuition, following the physiological function of the processes noted to be abnormal. This approach could leave physicians well-equipped for early FND management during their limited initial consultation and follow-up time (Table 5).
 Click to view | Table 5. Examples of Pathophysiologically Oriented, Intuitive Interventions, and Their Management Impact, Performed During Clinical Interview and Physical Examination |
Multi-specialty expertise and future recommendations
A muti-network approach must tend to pathways within the brain and between the brain and body, addressing their interconnectedness [188], a concept highlighted since the times of Erasmus, Darwin, and Pace Descartes [189]. An ideal treatment protocol must also combine skills from the fields of neurology, psychiatry and psychology [190], an efficacious confluence, witnessed when studying the work of physicians specializing in neurology and psychiatry, such as Sigmund Freud, Stanley Cobb, or Mathew J Burke [191].
Neurologists, psychiatrists, psychologists, and physiotherapists following a comprehensive and integrative FND pathophysiological model, should jointly review multiple therapeutic modalities. These include physiotherapies, CBT, psychodynamic, paradoxical intention, mindfulness-based, psychoeducation, eclectic interventions, biofeedback, family, group therapy, hypnosis, dream work, emotionally focused, somatic, and psychedelic therapies. From these, experts must choose the most appropriate techniques to target the pathophysiological processes and subconscious dynamics permeating the cognitive, limbic, autonomic, and sensorimotor networks, causing FND - and perhaps even the umbrella of FDs. This approach could also overcome the management uncertainties derived from the arguments of lumping versus splitting FND subtypes [192], potentially offering a comprehensive, pathophysiologically targeted treatment for all FD.
The creation of a comprehensive, pathologically focused, multi-network treatment algorithm is not a simple task and must be designed through multidisciplinary collaborative efforts, overcoming the detrimental effects of medical specialists’ separateness in FND management.
In summary, the phases to achieve the design of a multi-network FND management algorithm are: 1) To compile all pathophysiological mechanisms and dysfunctional FND networks in a single model; 2) To determine the best assessment procedures; 3) To choose the best techniques from the fields of neurology, psychiatry, psychology, and physiotherapy, capable of targeting limbic, cognitive, autonomic, and somatic subconscious dynamics; and 4) To design in-office interventions and homework exercises for patients.
Multi-specialty training for FND identification and management
Most specialists, especially PCP as the gateway to the healthcare system, are left uninformed as to how to help the FND population at first encounter. Authors [193-198] speak about the iatrogenic harm, unnecessary, costly evaluations, and unwarranted pharmacotherapies impacting the FND population, suggesting that early and appropriate interventions are needed.
Research has shown that an interdisciplinary approach providing early mental health services to families is beneficial [199, 200], and that standardized FND treatment reduces hospital length of stay, subspecialist consultations, and overall health care costs [201]. Programs utilizing multidisciplinary interventions for FND have demonstrated positive results [130, 202-204], although such treatment is most often available only to the minority of patients capable of reaching academic healthcare settings.
A comprehensive management, preventive and educational tool, capable of presenting multi-network interventions to professionals, patients, and family members is supported by FND pathophysiology. A unified assessment, treatment and educational algorithm could also serve as systematic training for the upcoming generations of healthcare professionals.
In summary, major knowledge gaps identified in this review include: 1) The official FND nomenclature contains the word “symptom” and the term “conversion”. 2) The absence of a pathophysiologically focused classification system for the umbrella of FD. 3) A biographically focused, biopsychosocial-spiritual assessment and management for FND, that investigates pertinent history from the prenatal period to the moment of each evaluation, has not been developed. 4) All the pathophysiological mechanisms involved in FND production and maintenance have not been reviewed, nor summarized in a single model. 5) A practical multi-network FND assessment and management algorithm capable of targeting subconscious dynamics in the neo-cortex, limbic, autonomic, and sensorimotor networks is missing. 6) An integrative FND pathophysiological assessment, management, and preventive multi-network management protocol is not available across medical specialties.
| Conclusions | ▴Top |
There are hundreds of epidemiological, genetic, clinical, and functional imaging studies demonstrating the dysfunctional neural regions and pathways associated with FND and other FD. Now is the time to design a biographically focused multi-network assessment, treatment, and prevention algorithm capable of targeting the pathophysiological mechanisms associated with FND. Treatment must go beyond CBT and CBT-predominant physiotherapies to comprehensively address and target the many networks participating in the production and maintenance of FND. Such an endeavor will allow us to make progress treating, as well as hopefully preventing, this increasingly frequent, expensive, and sometimes fatal neurological disorder.
Acknowledgments
Thanks to J. Christopher Edgar, PhD for reviewing and commenting on a draft of this paper. The authors confirm that the content has been presented a brief abstract in the Proceedings of the Functional Neurological Disorder Society 2022 Meeting.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Author Contributions
Yadira Velazquez-Rodriguez conceived, designed, researched the data, and drafted the article. Yadira Velazquez-Rodriguez and Brooke Fehily analyzed and interpreted the data. Both authors gave final approval of the version to be published.
Data Availability
There are no other related research objects (data, methods, protocols, software, hardware) to publish alongside this review article.
Abbreviations
FND: functional neurological disorder; ICD: International Classification of Diseases; DSM: Diagnostic and Statistical Manual of Mental Disorders; QOL: quality of life; FDs: functional disorders; FRDs: functional rheumatological disorders; FGIDs: functional gastrointestinal disorders; FCVD: functional cardiovascular disease; PTSD: post-traumatic stress disorder; FS: functional seizures; FE: functional epilepsy; PT: physical therapy; CBT: cognitive behavioral therapy; RCTs: randomized controlled trials; SMC: standardized medical care; CBT-ip: CBT-informed psychotherapy; CODES: Cognitive Behavioral Therapy vs. Standardized Medical Care for Adults with Dissociative Non-Epileptic Seizures; ReACT: retraining and control therapy; HPA: hypothalamic-pituitary-adrenal; ANS: autonomic nervous system; PCP: primary care physician
| References | ▴Top |
- Micale MS. Approaching hysteria: disease and its interpretations. Princeton University Press; 2019.
- Sigerist HE. A history of medicine: early Greek, Hindu, and Persian medicine. New York: Oxford University Press; 1951.
- Charlton TL, Short C. A Latin dictionary. Oxford: Clarendon Press; 1879.
- Eisen J. Suppressed inventions. Penguin; 2001.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 2nd Edition (DSM-II) ed: American Psychiatric Press, Washington, DC.; 1968.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd Edition (DSM-III) ed: American Psychiatric Press, Washington, DC.; 1980.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th Edition (DSM-IV) ed: American Psychiatric Press, Washington, DC.; 1994.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th Edition (DSM-V) ed: American Psychiatric Press, Washington, DC.; 2013.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th Edition (DSM-V-TR) ed: American Psychiatric Press, Washington, DC.; 2022.
- Carson A, Hallett M, Stone J. Assessment of patients with functional neurologic disorders. Handb Clin Neurol. 2016;139:169-188.
doi pubmed - Hallett M, Aybek S, Dworetzky BA, McWhirter L, Staab JP, Stone J. Functional neurological disorder: new subtypes and shared mechanisms. Lancet Neurol. 2022;21(6):537-550.
doi pubmed pmc - Yakhnitsa V, Ji G, Hein M, Presto P, Griffin Z, Ponomareva O, Navratilova E, et al. Kappa opioid receptor blockade in the amygdala mitigates pain like-behaviors by inhibiting corticotropin releasing factor neurons in a rat model of functional pain. Front Pharmacol. 2022;13:903978.
doi pubmed pmc - Hadjikhani N, Ward N, Boshyan J, Napadow V, Maeda Y, Truini A, Caramia F, et al. The missing link: enhanced functional connectivity between amygdala and visceroceptive cortex in migraine. Cephalalgia. 2013;33(15):1264-1268.
doi pubmed pmc - Maleki N, Becerra L, Brawn J, Bigal M, Burstein R, Borsook D. Concurrent functional and structural cortical alterations in migraine. Cephalalgia. 2012;32(8):607-620.
doi pubmed pmc - Morgante F, Matinella A, Andrenelli E, Ricciardi L, Allegra C, Terranova C, Girlanda P, et al. Pain processing in functional and idiopathic dystonia: An exploratory study. Mov Disord. 2018;33(8):1340-1348.
doi pubmed - Gelauff JM, Rosmalen JGM, Gardien J, Stone J, Tijssen MAJ. Shared demographics and comorbidities in different functional motor disorders. Parkinsonism Relat Disord. 2020;70:1-6.
doi pubmed - Tinazzi M, Morgante F, Marcuzzo E, Erro R, Barone P, Ceravolo R, Mazzucchi S, et al. Clinical Correlates of Functional Motor Disorders: An Italian Multicenter Study. Mov Disord Clin Pract. 2020;7(8):920-929.
doi pubmed pmc - Butler M, Shipston-Sharman O, Seynaeve M, Bao J, Pick S, Bradley-Westguard A, Ilola E, et al. International online survey of 1048 individuals with functional neurological disorder. Eur J Neurol. 2021;28(11):3591-3602.
doi pubmed - Kranick S, Ekanayake V, Martinez V, Ameli R, Hallett M, Voon V. Psychopathology and psychogenic movement disorders. Mov Disord. 2011;26(10):1844-1850.
doi pubmed pmc - Matin N, Young SS, Williams B, LaFrance WC, Jr., King JN, Caplan D, Chemali Z, et al. Neuropsychiatric associations with gender, illness duration, work disability, and motor subtype in a U.S. functional neurological disorders clinic population. J Neuropsychiatry Clin Neurosci. 2017;29(4):375-382.
doi pubmed - Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain. 2010;133(Pt 5):1537-1551.
doi pubmed - Diprose W, Sundram F, Menkes DB. Psychiatric comorbidity in psychogenic nonepileptic seizures compared with epilepsy. Epilepsy Behav. 2016;56:123-130.
doi pubmed - Walsh S, Levita L, Reuber M. Comorbid depression and associated factors in PNES versus epilepsy: Systematic review and meta-analysis. Seizure. 2018;60:44-56.
doi pubmed - Gelauff JM, Kingma EM, Kalkman JS, Bezemer R, van Engelen BGM, Stone J, Tijssen MAJ, et al. Fatigue, not self-rated motor symptom severity, affects quality of life in functional motor disorders. J Neurol. 2018;265(8):1803-1809.
doi pubmed - Jones B, Reuber M, Norman P. Correlates of health-related quality of life in adults with psychogenic nonepileptic seizures: A systematic review. Epilepsia. 2016;57(2):171-181.
doi pubmed - LaFrance WC, Jr., Friedman JH. Cognitive behavioral therapy for psychogenic movement disorder. Mov Disord. 2009;24(12):1856-1857.
doi pubmed - Gelauff J, Stone J, Edwards M, Carson A. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry. 2014;85(2):220-226.
doi pubmed - Glass SP, Matin N, Williams B, Mello J, Stephen CD, Young SS, Callahan J, et al. Neuropsychiatric factors linked to adherence and short-term outcome in a U.S. functional neurological disorders clinic: a retrospective cohort study. J Neuropsychiatry Clin Neurosci. 2018;30(2):152-159.
doi pubmed - Stone J. The bare essentials: Functional symptoms in neurology. Pract Neurol. 2009;9(3):179-189.
doi pubmed - Morsy SK, Aybek S, Carson A, Nicholson TR, Stone J, Kamal AM, et al. The relationship between types of life events and the onset of functional neurological (conversion) disorder in adults: a systematic review and meta-analysis. Psychological Medicine. 2021:1-18.
- Brown RJ. Dissociation and conversion in psychogenic illness. In: Hallett M, Fahn S, Jankovic J, Lang AE, Cloninger CR, Yudofsky SC, editors. Psychogenic movement disorders: Neurology and neuropsychiatry. Lippincott Williams & Wilkins Publishers; 2006. p. 131-143.
- Brown RJ. Explaining the unexplained. The Psychologist. 2013.
- Brown RJ. Dissociation and somatoform disorders. In: Kennedy FC, Kennerley H, Pearson DG, editors. Cognitive behavioural approaches to the understanding and treatment of dissociation. Routledge London; 2013.
- Stamelou M, Cossu G, Edwards MJ, Murgia D, Parees I, Melis M, Bhatia KP. Familial psychogenic movement disorders. Mov Disord. 2013;28(9):1295-1298.
doi pubmed - Roelofs K, Keijsers GP, Hoogduin KA, Naring GW, Moene FC. Childhood abuse in patients with conversion disorder. Am J Psychiatry. 2002;159(11):1908-1913.
doi pubmed - Stone J, Warlow C, Deary I, Sharpe M. Predisposing Risk Factors for Functional Limb Weakness: A Case-Control Study. J Neuropsychiatry Clin Neurosci. 2020;32(1):50-57.
doi pubmed - Fiszman A, Alves-Leon SV, Nunes RG, D'Andrea I, Figueira I. Traumatic events and posttraumatic stress disorder in patients with psychogenic nonepileptic seizures: a critical review. Epilepsy Behav. 2004;5(6):818-825.
doi pubmed - Roelofs K, Spinhoven P. Trauma and medically unexplained symptoms towards an integration of cognitive and neuro-biological accounts. Clin Psychol Rev. 2007;27(7):798-820.
doi pubmed - Sharpe D, Faye C. Non-epileptic seizures and child sexual abuse: a critical review of the literature. Clin Psychol Rev. 2006;26(8):1020-1040.
doi pubmed - Nimmo-Smith V, Brugha TS, Kerr MP, McManus S, Rai D. Discrimination, domestic violence, abuse, and other adverse life events in people with epilepsy: Population-based study to assess the burden of these events and their contribution to psychopathology. Epilepsia. 2016;57(11):1870-1878.
doi pubmed - Stager L, Morriss S, Szaflarski JP, Fobian AD. Psychiatric and personality factors in pediatric functional seizures: A prospective case-control study. Seizure. 2022;98:105-112.
doi pubmed pmc - Hansen AS, Rask CU, Christensen AE, Rodrigo-Domingo M, Christensen J, Nielsen RE. Psychiatric disorders in children and adolescents with psychogenic nonepileptic seizures. Neurology. 2021;97(5):e464-e475.
doi pubmed - Carson A, Stone J, Hibberd C, Murray G, Duncan R, Coleman R, Warlow C, et al. Disability, distress and unemployment in neurology outpatients with symptoms 'unexplained by organic disease'. J Neurol Neurosurg Psychiatry. 2011;82(7):810-813.
doi pubmed - Carson AJ, Ringbauer B, Stone J, McKenzie L, Warlow C, Sharpe M. Do medically unexplained symptoms matter? A prospective cohort study of 300 new referrals to neurology outpatient clinics. J Neurol Neurosurg Psychiatry. 2000;68(2):207-210.
doi pubmed pmc - Feinstein A, Stergiopoulos V, Fine J, Lang AE. Psychiatric outcome in patients with a psychogenic movement disorder: a prospective study. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14(3):169-176.
pubmed - Carson A, Lehn A. Epidemiology. Handb Clin Neurol. 2016;139:47-60.
doi pubmed - Binzer M, Andersen PM, Kullgren G. Clinical characteristics of patients with motor disability due to conversion disorder: a prospective control group study. J Neurol Neurosurg Psychiatry. 1997;63(1):83-88.
doi pubmed pmc - Stefansson JG, Messina JA, Meyerowitz S. Hysterical neurosis, conversion type: clinical and epidemiological considerations. Acta Psychiatr Scand. 1976;53(2):119-138.
doi pubmed - Stevens DL. Neurology in Gloucestershire: the clinical workload of an English neurologist. J Neurol Neurosurg Psychiatry. 1989;52(4):439-446.
doi pubmed pmc - Akagi H, House A. The clinical epidemiology of hysteria: vanishingly rare, or just vanishing? Psychol Med. 2002;32(2):191-194.
doi pubmed - Stone J, Carson A, Duncan R, Roberts R, Warlow C, Hibberd C, Coleman R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112(9):747-751.
doi pubmed - Khoja LA, Coebergh J, Nicholson TR. 28 The link between functional neurological disorder (FND) & migraine: a systematic review. BMJ Publishing Group Ltd; 2020.
- Ercoli T, Lutzoni L, Orofino G, Muroni A, Defazio G. Functional neurological disorder after COVID-19 vaccination. Neurol Sci. 2021;42(10):3989-3990.
doi pubmed pmc - Butler M, Coebergh J, Safavi F, Carson A, Hallett M, Michael B, Pollak TA, et al. Functional neurological disorder after SARS-CoV-2 vaccines: two case reports and discussion of potential public health implications. J Neuropsychiatry Clin Neurosci. 2021;33(4):345-348.
doi pubmed pmc - Fasano A, Daniele A. Functional disorders after COVID-19 vaccine fuel vaccination hesitancy. J Neurol Neurosurg Psychiatry. 2022;93(3):339-340.
doi pubmed - Taylor S, Asmundson GJG. Immunization stress-related responses: Implications for vaccination hesitancy and vaccination processes during the COVID-19 pandemic. J Anxiety Disord. 2021;84:102489.
doi pubmed pmc - Hull M, Parnes M. Tics and TikTok: Functional Tics Spread Through Social Media. Mov Disord Clin Pract. 2021;8(8):1248-1252.
doi pubmed pmc - Hansen AS. Psychogenic nonepileptic seizures in children and adolescents. Incidence, characteristics and morbidity in a Danish nationwide study. 2020.
- Raper J, Currigan V, Fothergill S, Stone J, Forsyth RJ. Long-term outcomes of functional neurological disorder in children. Arch Dis Child. 2019;104(12):1155-1160.
doi pubmed - Nightscales R, McCartney L, Auvrez C, Tao G, Barnard S, Malpas CB, Perucca P, et al. Mortality in patients with psychogenic nonepileptic seizures. Neurology. 2020;95(6):e643-e652.
doi pubmed - Zhang L, Beghi E, Tomson T, Beghi M, Erba G, Chang Z. Mortality in patients with psychogenic non-epileptic seizures a population-based cohort study. J Neurol Neurosurg Psychiatry. 2022;93(4):379-385.
doi pubmed - Jennum P, Ibsen R, Kjellberg J. Morbidity and mortality of nonepileptic seizures (NES): A controlled national study. Epilepsy Behav. 2019;96:229-233.
doi pubmed - Crump C, Ioannidis JP, Sundquist K, Winkleby MA, Sundquist J. Mortality in persons with mental disorders is substantially overestimated using inpatient psychiatric diagnoses. J Psychiatr Res. 2013;47(10):1298-1303.
doi pubmed pmc - Fazel S, Wolf A, Langstrom N, Newton CR, Lichtenstein P. Premature mortality in epilepsy and the role of psychiatric comorbidity: a total population study. Lancet. 2013;382(9905):1646-1654.
doi pubmed pmc - Galimberti CA, Ratti MT, Murelli R, Marchioni E, Manni R, Tartara A. Patients with psychogenic nonepileptic seizures, alone or epilepsy-associated, share a psychological profile distinct from that of epilepsy patients. J Neurol. 2003;250(3):338-346.
doi pubmed - Seneviratne U, Briggs B, Lowenstern D, D'Souza W. The spectrum of psychogenic non-epileptic seizures and comorbidities seen in an epilepsy monitoring unit. J Clin Neurosci. 2011;18(3):361-363.
doi pubmed - Seneviratne U, Low ZM, Low ZX, Hehir A, Paramaswaran S, Foong M, Ma H, et al. Medical health care utilization cost of patients presenting with psychogenic nonepileptic seizures. Epilepsia. 2019;60(2):349-357.
doi pubmed - Begley CE, Annegers JF, Lairson DR, Reynolds TF, Hauser WA. Cost of epilepsy in the United States: a model based on incidence and prognosis. Epilepsia. 1994;35(6):1230-1243.
doi pubmed - Barsky AJ, Orav EJ, Bates DW. Somatization increases medical utilization and costs independent of psychiatric and medical comorbidity. Arch Gen Psychiatry. 2005;62(8):903-910.
doi pubmed - Crimlisk HL, Bhatia KP, Cope H, David AS, Marsden D, Ron MA. Patterns of referral in patients with medically unexplained motor symptoms. J Psychosom Res. 2000;49(3):217-219.
doi pubmed - Goldstein LH, Robinson EJ, Mellers JDC, Stone J, Carson A, Chalder T, Reuber M, et al. Psychological and demographic characteristics of 368 patients with dissociative seizures: data from the CODES cohort. Psychol Med. 2021;51(14):2433-2445.
doi pubmed pmc - Jennum P, Ibsen R, Kjellberg J. Welfare consequences for people diagnosed with nonepileptic seizures: A matched nationwide study in Denmark. Epilepsy Behav. 2019;98(Pt A):59-65.
doi pubmed - Robson C, Myers L, Pretorius C, Lian OS, Reuber M. Health related quality of life of people with non-epileptic seizures: The role of socio-demographic characteristics and stigma. Seizure. 2018;55:93-99.
doi pubmed pmc - Szaflarski JP, Szaflarski M. Seizure disorders, depression, and health-related quality of life. Epilepsy Behav. 2004;5(1):50-57.
doi pubmed - Pugliatti M, Beghi E, Forsgren L, Ekman M, Sobocki P. Estimating the cost of epilepsy in Europe: a review with economic modeling. Epilepsia. 2007;48(12):2224-2233.
doi pubmed - Riechmann J, Strzelczyk A, Reese JP, Boor R, Stephani U, Langner C, Neubauer BA, et al. Costs of epilepsy and cost-driving factors in children, adolescents, and their caregivers in Germany. Epilepsia. 2015;56(9):1388-1397.
doi pubmed - Strzelczyk A, Reese JP, Dodel R, Hamer HM. Cost of epilepsy: a systematic review. Pharmacoeconomics. 2008;26(6):463-476.
doi pubmed - Popkirov S, Nicholson TR, Bloem BR, Cock HR, Derry CP, Duncan R, Dworetzky BA, et al. Hiding in plain sight: functional neurological disorders in the news. J Neuropsychiatry Clin Neurosci. 2019;31(4):361-367.
doi pubmed pmc - Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129-136.
doi pubmed - Saxena A, Paredes-Echeverri S, Michaelis R, Popkirov S, Perez DL. Using the Biopsychosocial Model to Guide Patient-Centered Neurological Treatments. Semin Neurol. 2022;42(2):80-87.
doi pubmed - Deuschl G, Koster B, Lucking CH, Scheidt C. Diagnostic and pathophysiological aspects of psychogenic tremors. Mov Disord. 1998;13(2):294-302.
doi pubmed - Kim YJ, Pakiam AS, Lang AE. Historical and clinical features of psychogenic tremor: a review of 70 cases. Can J Neurol Sci. 1999;26(3):190-195.
doi pubmed - Ljungberg L. Hysteria; a clinical, prognostic and genetic study. Acta Psychiatr Neurol Scand Suppl. 1957;112:1-162.
pubmed - Thomas M, Vuong KD, Jankovic J. Long-term prognosis of patients with psychogenic movement disorders. Parkinsonism Relat Disord. 2006;12(6):382-387.
doi pubmed - Carson AJ, Best S, Postma K, Stone J, Warlow C, Sharpe M. The outcome of neurology outpatients with medically unexplained symptoms: a prospective cohort study. J Neurol Neurosurg Psychiatry. 2003;74(7):897-900.
doi pubmed pmc - Sharpe M, Stone J, Hibberd C, Warlow C, Duncan R, Coleman R, Roberts R, et al. Neurology out-patients with symptoms unexplained by disease: illness beliefs and financial benefits predict 1-year outcome. Psychol Med. 2010;40(4):689-698.
doi pubmed - Binzer M, Kullgren G. Motor conversion disorder. A prospective 2- to 5-year follow-up study. Psychosomatics. 1998;39(6):519-527.
doi pubmed - Stone J, Sharpe M, Rothwell PM, Warlow CP. The 12 year prognosis of unilateral functional weakness and sensory disturbance. J Neurol Neurosurg Psychiatry. 2003;74(5):591-596.
doi pubmed pmc - Durrant J, Rickards H, Cavanna AE. Prognosis and outcome predictors in psychogenic nonepileptic seizures. Epilepsy Res Treat. 2011;2011:274736.
doi pubmed pmc - Edwards MJ, Fotopoulou A, Parees I. Neurobiology of functional (psychogenic) movement disorders. Curr Opin Neurol. 2013;26(4):442-447.
doi pubmed pmc - Homberg V. Neurorehabilitation approaches to facilitate motor recovery. Handb Clin Neurol. 2013;110:161-173.
doi pubmed - Speed J. Behavioral management of conversion disorder: retrospective study. Arch Phys Med Rehabil. 1996;77(2):147-154.
doi pubmed - Nielsen G, Stone J, Matthews A, Brown M, Sparkes C, Farmer R, Masterton L, et al. Physiotherapy for functional motor disorders: a consensus recommendation. J Neurol Neurosurg Psychiatry. 2015;86(10):1113-1119.
doi pubmed pmc - Dallocchio C, Arbasino C, Klersy C, Marchioni E. The effects of physical activity on psychogenic movement disorders. Mov Disord. 2010;25(4):421-425.
doi pubmed - Jordbru AA, Smedstad LM, Klungsoyr O, Martinsen EW. Psychogenic gait disorder: a randomized controlled trial of physical rehabilitation with one-year follow-up. J Rehabil Med. 2014;46(2):181-187.
doi pubmed - Nielsen G, Stone J, Edwards MJ. Physiotherapy for functional (psychogenic) motor symptoms: a systematic review. J Psychosom Res. 2013;75(2):93-102.
doi pubmed - Nielsen G, Buszewicz M, Stevenson F, Hunter R, Holt K, Dudziec M, Ricciardi L, et al. Randomised feasibility study of physiotherapy for patients with functional motor symptoms. J Neurol Neurosurg Psychiatry. 2017;88(6):484-490.
doi pubmed - Baker J, Barnett C, Cavalli L, Dietrich M, Dixon L, Duffy JR, Elias A, et al. Management of functional communication, swallowing, cough and related disorders: consensus recommendations for speech and language therapy. J Neurol Neurosurg Psychiatry. 2021;92(10):1112-1125.
doi pubmed - Nicholson C, Edwards MJ, Carson AJ, Gardiner P, Golder D, Hayward K, Humblestone S, et al. Occupational therapy consensus recommendations for functional neurological disorder. J Neurol Neurosurg Psychiatry. 2020;91(10):1037-1045.
doi pubmed - Morgante F, Edwards MJ, Espay AJ. Psychogenic movement disorders. Continuum (Minneap Minn). 2013;19(5):1383-1396.
doi pubmed pmc - Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med. 2007;69(9):881-888.
doi pubmed - Goldstein LH, Deale AC, Mitchell-O'Malley SJ, Toone BK, Mellers JD. An evaluation of cognitive behavioral therapy as a treatment for dissociative seizures: a pilot study. Cogn Behav Neurol. 2004;17(1):41-49.
doi pubmed - LaFrance WC, Jr., Baird GL, Barry JJ, Blum AS, Frank Webb A, Keitner GI, Machan JT, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry. 2014;71(9):997-1005.
doi pubmed - Goldstein LH, Chalder T, Chigwedere C, Khondoker MR, Moriarty J, Toone BK, Mellers JD. Cognitive-behavioral therapy for psychogenic nonepileptic seizures: a pilot RCT. Neurology. 2010;74(24):1986-1994.
doi pubmed pmc - Myers L, Vaidya-Mathur U, Lancman M. Prolonged exposure therapy for the treatment of patients diagnosed with psychogenic non-epileptic seizures (PNES) and post-traumatic stress disorder (PTSD). Epilepsy Behav. 2017;66:86-92.
doi pubmed - Deleuran M, Norgaard K, Andersen NB, Sabers A. Psychogenic nonepileptic seizures treated with psychotherapy: Long-term outcome on seizures and healthcare utilization. Epilepsy Behav. 2019;98(Pt A):195-200.
doi pubmed - Espay AJ, Ries S, Maloney T, Vannest J, Neefus E, Dwivedi AK, Allendorfer JB, et al. Clinical and neural responses to cognitive behavioral therapy for functional tremor. Neurology. 2019;93(19):e1787-e1798.
doi pubmed pmc - Goldstein LH, Mellers JD, Landau S, Stone J, Carson A, Medford N, Reuber M, et al. COgnitive behavioural therapy vs standardised medical care for adults with Dissociative non-Epileptic Seizures (CODES): a multicentre randomised controlled trial protocol. BMC Neurol. 2015;15:98.
doi pubmed pmc - Goldstein LH, Robinson EJ, Chalder T, Stone J, Reuber M, Medford N, Carson A, et al. Moderators of cognitive behavioural therapy treatment effects and predictors of outcome in the CODES randomised controlled trial for adults with dissociative seizures. J Psychosom Res. 2022;158:110921.
doi pubmed - Rawat VS, Dhiman V, Sinha S, Vijay Sagar KJ, Thippeswamy H, Chaturvedi SK, Srinath S, et al. Co-morbidities and outcome of childhood psychogenic non-epileptic seizures—an observational study. Seizure. 2015;25:95-98.
doi pubmed - Terry D, Enciso L, Trott K, Burch MM, Albert DVF. Outcomes in children and adolescents with psychogenic nonepileptic events using a multidisciplinary clinic approach. J Child Neurol. 2020;35(13):918-923.
doi pubmed - Fredwall M, Terry D, Enciso L, Burch MM, Trott K, Albert DVF. Outcomes of children and adolescents 1 year after being seen in a multidisciplinary psychogenic nonepileptic seizures clinic. Epilepsia. 2021;62(10):2528-2538.
doi pubmed - Fobian AD, Long DM, Szaflarski JP. Retraining and control therapy for pediatric psychogenic non-epileptic seizures. Ann Clin Transl Neurol. 2020;7(8):1410-1419.
doi pubmed pmc - Yadav A, Agarwal R, Park J. Outcome of psychogenic nonepileptic seizures (PNES) in children: A 2-year follow-up study. Epilepsy Behav. 2015;53:168-173.
doi pubmed - Carson A, Ludwig L, Welch K. Psychologic theories in functional neurologic disorders. Handb Clin Neurol. 2016;139:105-120.
doi pubmed - Carlson P, Nicholson Perry K. Psychological interventions for psychogenic non-epileptic seizures: A meta-analysis. Seizure. 2017;45:142-150.
doi pubmed - Kompoliti K, Wilson B, Stebbins G, Bernard B, Hinson V. Immediate vs. delayed treatment of psychogenic movement disorders with short term psychodynamic psychotherapy: randomized clinical trial. Parkinsonism Relat Disord. 2014;20(1):60-63.
doi pubmed - Hinson VK, Weinstein S, Bernard B, Leurgans SE, Goetz CG. Single-blind clinical trial of psychotherapy for treatment of psychogenic movement disorders. Parkinsonism Relat Disord. 2006;12(3):177-180.
doi pubmed - Mayor R, Howlett S, Grunewald R, Reuber M. Long-term outcome of brief augmented psychodynamic interpersonal therapy for psychogenic nonepileptic seizures: seizure control and health care utilization. Epilepsia. 2010;51(7):1169-1176.
doi pubmed - Barry JJ, Wittenberg D, Bullock KD, Michaels JB, Classen CC, Fisher RS. Group therapy for patients with psychogenic nonepileptic seizures: a pilot study. Epilepsy Behav. 2008;13(4):624-629.
doi pubmed - Brown RJ, Reuber M. Psychological and psychiatric aspects of psychogenic non-epileptic seizures (PNES): A systematic review. Clin Psychol Rev. 2016;45:157-182.
doi pubmed - Asadi-Pooya AA, Brigo F, Kozlowska K, Perez DL, Pretorius C, Sawchuk T, Saxena A, et al. Social aspects of life in patients with functional seizures: Closing the gap in the biopsychosocial formulation. Epilepsy Behav. 2021;117:107903.
doi pubmed - Jungilligens J, Paredes-Echeverri S, Popkirov S, Barrett LF, Perez DL. A new science of emotion: implications for functional neurological disorder. Brain. 2022;145(8):2648-2663.
doi pubmed pmc - Baslet G, Dworetzky BA, Morrissey L, Ridlon R, Latawiec M, editors. Sustained improvement after completion of mindfulness-based psychotherapy for psychogenic nonepileptic seizures. Journal of Neuropsychiatry and Clinical Neurosciences; 2021: Amer Psychiatric Publishing, Inc 800 Maine Ave Sw, Suite 900, Washington, DC.
- Baslet G, Ehlert A, Oser M, Dworetzky BA. Mindfulness-based therapy for psychogenic nonepileptic seizures. Epilepsy Behav. 2020;103(Pt A):106534.
doi pubmed - LaFrance WC, Jr., Reuber M, Goldstein LH. Management of psychogenic nonepileptic seizures. Epilepsia. 2013;54(Suppl 1):53-67.
doi pubmed - Kozlowska K, Chudleigh C, Cruz C, Lim M, McClure G, Savage B, Shah U, et al. Psychogenic non-epileptic seizures in children and adolescents: Part II - explanations to families, treatment, and group outcomes. Clin Child Psychol Psychiatry. 2018;23(1):160-176.
doi pubmed pmc - Kozlowska K, Palmer DM, Brown KJ, McLean L, Scher S, Gevirtz R, Chudleigh C, et al. Reduction of autonomic regulation in children and adolescents with conversion disorders. Psychosom Med. 2015;77(4):356-370.
doi pubmed - Kozlowska K, Rampersad R, Cruz C, Shah U, Chudleigh C, Soe S, Gill D, et al. The respiratory control of carbon dioxide in children and adolescents referred for treatment of psychogenic non-epileptic seizures. Eur Child Adolesc Psychiatry. 2017;26(10):1207-1217.
doi pubmed pmc - Kozlowska K, Chudleigh C, Elliott B, Landini A. The body comes to family therapy: Treatment of a school-aged boy with hyperventilation-induced non-epileptic seizures. Clin Child Psychol Psychiatry. 2016;21(4):669-685.
doi pubmed - Chinta SS, Malhi P, Singhi P, Prabhakar S. Clinical and psychosocial characteristics of children with nonepileptic seizures. Ann Indian Acad Neurol. 2008;11(3):159-163.
doi pubmed pmc - Milan-Tomas A, Persyko M, Del Campo M, Shapiro CM, Farcnik K. An overview of psychogenic non-epileptic seizures: etiology, diagnosis and management. Can J Neurol Sci. 2018;45(2):130-136.
doi pubmed - Sawchuk T, Buchhalter J. Psychogenic nonepileptic seizures in children - Psychological presentation, treatment, and short-term outcomes. Epilepsy Behav. 2015;52(Pt A):49-56.
doi pubmed - Kozlowska K, English M, Savage B, Chudleigh C. Multimodal rehabilitation: a mind-body, family-based intervention for children and adolescents impaired by medically unexplained symptoms. Part 1: the program. The American Journal of Family Therapy. 2012;40(5):399-419.
- Doss JL, Plioplys S. Pediatric psychogenic nonepileptic seizures: a concise review. Child Adolesc Psychiatr Clin N Am. 2018;27(1):53-61.
doi pubmed - Sawchuk T, Buchhalter J, Senft B. Psychogenic nonepileptic seizures in children-Prospective validation of a clinical care pathway & risk factors for treatment outcome. Epilepsy Behav. 2020;105:106971.
doi pubmed - Hunter EC, Charlton J, David AS. Depersonalisation and derealisation: assessment and management. BMJ. 2017;356:j745.
doi pubmed - Carter A, Denton A, Ladino LD, Hassan I, Sawchuk T, Snyder T, Vrbancic M, et al. Experience of psychogenic nonepileptic seizures in the Canadian league against epilepsy: A survey describing current practices by neurologists and epileptologists. Seizure. 2018;61:227-233.
doi pubmed - Gasparini S, Beghi E, Ferlazzo E, Beghi M, Belcastro V, Biermann KP, Bottini G, et al. Management of psychogenic non-epileptic seizures: a multidisciplinary approach. Eur J Neurol. 2019;26(2):205-e215.
doi pubmed - Jacob AE, Kaelin DL, Roach AR, Ziegler CH, LaFaver K. Motor retraining (MoRe) for functional movement disorders: outcomes from a 1-week multidisciplinary rehabilitation program. PM R. 2018;10(11):1164-1172.
doi pubmed - LaFaver K, LaFrance WC, Price ME, Rosen PB, Rapaport M. Treatment of functional neurological disorder: current state, future directions, and a research agenda. CNS Spectr. 2020:1-7.
doi pubmed - Gelauff JM, Carson A, Ludwig L, Tijssen MAJ, Stone J. The prognosis of functional limb weakness: a 14-year case-control study. Brain. 2019;142(7):2137-2148.
doi pubmed - McCormack R, Moriarty J, Mellers JD, Shotbolt P, Pastena R, Landes N, Goldstein L, et al. Specialist inpatient treatment for severe motor conversion disorder: a retrospective comparative study. J Neurol Neurosurg Psychiatry. 2014;85(8):895-900.
doi pubmed - Saifee TA, Kassavetis P, Parees I, Kojovic M, Fisher L, Morton L, Foong J, et al. Inpatient treatment of functional motor symptoms: a long-term follow-up study. J Neurol. 2012;259(9):1958-1963.
doi pubmed - Espay AJ, Aybek S, Carson A, Edwards MJ, Goldstein LH, Hallett M, LaFaver K, et al. Current concepts in diagnosis and treatment of functional neurological disorders. JAMA Neurol. 2018;75(9):1132-1141.
doi pubmed pmc - Tilahun BBS, Thompson NR, Sankary LR, Laryea F, Trunick CM, Jehi LE. Outcomes in the treatment of psychogenic nonepileptic seizures (PNES) with CBTip: Response in seizure frequency, depression, anxiety, and quality of life. Epilepsy Behav. 2021;123:108277.
doi pubmed - LaFrance WC, Jr., Ho WLN, Bhatla A, Baird GL, Altalib HH, Godleski L. Treatment of psychogenic nonepileptic seizures (PNES) using video telehealth. Epilepsia. 2020;61(11):2572-2582.
doi pubmed - Demartini B, Bombieri F, Goeta D, Gambini O, Ricciardi L, Tinazzi M. A physical therapy programme for functional motor symptoms: A telemedicine pilot study. Parkinsonism Relat Disord. 2020;76:108-111.
doi pubmed - Pick S, Anderson DG, Asadi-Pooya AA, Aybek S, Baslet G, Bloem BR, Bradley-Westguard A, et al. Outcome measurement in functional neurological disorder: a systematic review and recommendations. J Neurol Neurosurg Psychiatry. 2020;91(6):638-649.
doi pubmed pmc - Nicholson TR, Carson A, Edwards MJ, Goldstein LH, Hallett M, Mildon B, Nielsen G, et al. Outcome measures for functional neurological disorder: a review of the theoretical complexities. J Neuropsychiatry Clin Neurosci. 2020;32(1):33-42.
doi pubmed - Roelofs K, Pasman J. Stress, childhood trauma, and cognitive functions in functional neurologic disorders. Handb Clin Neurol. 2016;139:139-155.
doi pubmed - Brown RJ. Dissociation and functional neurologic disorders. Handb Clin Neurol. 2016;139:85-94.
doi pubmed - Edwards MJ. Neurobiologic theories of functional neurologic disorders. Handb Clin Neurol. 2017;139:131-137.
doi pubmed - Deeley Q. Hypnosis as a model of functional neurologic disorders. Handb Clin Neurol. 2016;139:95-103.
doi pubmed - Drane DL, Fani N, Hallett M, Khalsa SS, Perez DL, Roberts NA. A framework for understanding the pathophysiology of functional neurological disorder. CNS Spectr. 2020:1-7.
doi pubmed pmc - Aybek S, Perez DL. Diagnosis and management of functional neurological disorder. BMJ. 2022;376:o64.
doi pubmed - Harvey AG, Watkins E, Mansell W. Cognitive behavioural processes across psychological disorders: A transdiagnostic approach to research and treatment. Oxford University Press, USA; 2004.
- Barlow DH, Allen LB, Choate ML. Toward a Unified Treatment for Emotional Disorders - Republished Article. Behav Ther. 2016;47(6):838-853.
doi pubmed - Gilmour G, Langer L, Bhatt H, Marcelle K, MacGillivray L, Lidstone S. Factors influencing triage to rehabilitation in functional movement disorder. Poster session presented at: 4th International Conference on Functional neurological Disorders. 2022 June 19-21; Boston, MA.
- Drane DL, Locke DE. Mechanisms of possible neurocognitive dysfunction. Oxford University Press New York; 2017.
- Dworetzky BA, Baslet G. Mechanisms of possible neurocognitive dysfunction. Psychogenic Nonepileptic Seizures: Toward the Integration of Care: Oxford University Press; 2017.
- Aybek S, Nicholson TR, O'Daly O, Zelaya F, Kanaan RA, David AS. Emotion-motion interactions in conversion disorder: an FMRI study. PLoS One. 2015;10(4):e0123273.
doi pubmed pmc - Voon V, Brezing C, Gallea C, Ameli R, Roelofs K, LaFrance WC, Jr., Hallett M. Emotional stimuli and motor conversion disorder. Brain. 2010;133(Pt 5):1526-1536.
doi pubmed pmc - Vuilleumier P, Chicherio C, Assal F, Schwartz S, Slosman D, Landis T. Functional neuroanatomical correlates of hysterical sensorimotor loss. Brain. 2001;124(Pt 6):1077-1090.
doi pubmed - Savitz JB, van der Merwe L, Newman TK, Solms M, Stein DJ, Ramesar RS. The relationship between childhood abuse and dissociation. Is it influenced by catechol-O-methyltransferase (COMT) activity? Int J Neuropsychopharmacol. 2008;11(2):149-161.
doi pubmed - de Lange FP, Roelofs K, Toni I. Increased self-monitoring during imagined movements in conversion paralysis. Neuropsychologia. 2007;45(9):2051-2058.
doi pubmed - Cojan Y, Waber L, Carruzzo A, Vuilleumier P. Motor inhibition in hysterical conversion paralysis. Neuroimage. 2009;47(3):1026-1037.
doi pubmed - Cojan Y, Waber L, Schwartz S, Rossier L, Forster A, Vuilleumier P. The brain under self-control: modulation of inhibitory and monitoring cortical networks during hypnotic paralysis. Neuron. 2009;62(6):862-875.
doi pubmed - Roberts NA, Reuber M. Alterations of consciousness in psychogenic nonepileptic seizures: emotion, emotion regulation and dissociation. Epilepsy Behav. 2014;30:43-49.
doi pubmed - Kozlowska K. Functional somatic symptoms in childhood and adolescence. Curr Opin Psychiatry. 2013;26(5):485-492.
doi pubmed - Heim CM, Mayberg HS, Mletzko T, Nemeroff CB, Pruessner JC. Decreased cortical representation of genital somatosensory field after childhood sexual abuse. Am J Psychiatry. 2013;170(6):616-623.
doi pubmed - Griffith JL, Polles A, Griffith ME. Pseudoseizures, families, and unspeakable dilemmas. Psychosomatics. 1998;39(2):144-153.
doi pubmed - van der Kruijs SJ, Vonck KE, Langereis GR, Feijs LM, Bodde NM, Lazeron RH, Carrette E, et al. Autonomic nervous system functioning associated with psychogenic nonepileptic seizures: Analysis of heart rate variability. Epilepsy Behav. 2016;54:14-19.
doi pubmed - Goldstein LH, Mellers JD. Ictal symptoms of anxiety, avoidance behaviour, and dissociation in patients with dissociative seizures. J Neurol Neurosurg Psychiatry. 2006;77(5):616-621.
doi pubmed pmc - Kozlowska K. Healing the disembodied mind: contemporary models of conversion disorder. Harv Rev Psychiatry. 2005;13(1):1-13.
doi pubmed - Ponnusamy A, Marques JL, Reuber M. Comparison of heart rate variability parameters during complex partial seizures and psychogenic nonepileptic seizures. Epilepsia. 2012;53(8):1314-1321.
doi pubmed - Marshall JC, Halligan PW, Fink GR, Wade DT, Frackowiak RS. The functional anatomy of a hysterical paralysis. Cognition. 1997;64(1):B1-8.
doi pubmed - Brandt T, Dieterich M, Strupp M. Somatoform vertigo and dizziness syndromes. In Vertigo and Dizziness. Springer. 2013:153-164.
- Bogaerts K, Millen A, Li W, De Peuter S, Van Diest I, Vlemincx E, Fannes S, et al. High symptom reporters are less interoceptively accurate in a symptom-related context. J Psychosom Res. 2008;65(5):417-424.
doi pubmed - Deeley Q, Oakley DA, Walsh E, Bell V, Mehta MA, Halligan PW. Modelling psychiatric and cultural possession phenomena with suggestion and fMRI. Cortex. 2014;53:107-119.
doi pubmed - Voon V, Brezing C, Gallea C, Hallett M. Aberrant supplementary motor complex and limbic activity during motor preparation in motor conversion disorder. Mov Disord. 2011;26(13):2396-2403.
doi pubmed pmc - Bakvis P, Roelofs K, Kuyk J, Edelbroek PM, Swinkels WA, Spinhoven P. Trauma, stress, and preconscious threat processing in patients with psychogenic nonepileptic seizures. Epilepsia. 2009;50(5):1001-1011.
doi pubmed - Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162-1167.
doi pubmed - Voon V, Gallea C, Hattori N, Bruno M, Ekanayake V, Hallett M. The involuntary nature of conversion disorder. Neurology. 2010;74(3):223-228.
doi pubmed pmc - Bakvis P, Spinhoven P, Giltay EJ, Kuyk J, Edelbroek PM, Zitman FG, Roelofs K. Basal hypercortisolism and trauma in patients with psychogenic nonepileptic seizures. Epilepsia. 2010;51(5):752-759.
doi pubmed - Apazoglou K, Mazzola V, Wegrzyk J, Frasca Polara G, Aybek S. Biological and perceived stress in motor functional neurological disorders. Psychoneuroendocrinology. 2017;85:142-150.
doi pubmed - Edwards MJ. Functional neurological disorder: lighting the way to a new paradigm for medicine. Brain. 2021;144(11):3279-3282.
doi pubmed pmc - Yudofsky SC, Hales RE. Neuropsychiatry and the future of psychiatry and neurology. Am J Psychiatry. 2002;159(8):1261-1264.
doi pubmed - Trimble M. The intentional brain—a short history of neuropsychiatry. CNS Spectr. 2016;21(3):223-229.
doi pubmed - Begue I, Nicholson TR, Kozlowska K, LaFrance WC, Levenson JL, Rapaport MH, Carson AJ, et al. Psychiatry's modern role in functional neurological disorder: join the renaissance. Psychol Med. 2021;51(12):1961-1963.
doi pubmed - Burke MJ. "It's All in Your Head"-Medicine's Silent Epidemic. JAMA Neurol. 2019;76(12):1417-1418.
doi pubmed - Tinazzi M, Geroin C, Marcuzzo E, Cuoco S, Ceravolo R, Mazzucchi S, Pilotto A, et al. Functional motor phenotypes: to lump or to split? J Neurol. 2021;268(12):4737-4743.
doi pubmed pmc - Montague DK, Jones LR. Psychogenic urinary retention. Urology. 1979;13(1):30-35.
doi pubmed - Cardenas DD, Larson J, Egan KJ. Hysterical paralysis in the upper extremity of chronic pain patients. Arch Phys Med Rehabil. 1986;67(3):190-193.
doi pubmed - Jankovic J. Diagnosis and treatment of psychogenic parkinsonism. J Neurol Neurosurg Psychiatry. 2011;82(12):1300-1303.
doi pubmed - Schmerler DA, Espay AJ. Functional dystonia. Handb Clin Neurol. 2016;139:235-245.
doi pubmed - Kolbrunner J, Menet AD, Seifert E. Psychogenic aphonia: no fixation even after a lengthy period of aphonia. Swiss Med Wkly. 2010;140(1-2):12-17.
doi pubmed - Lee BE, Kim GH. Globus pharyngeus: a review of its etiology, diagnosis and treatment. World J Gastroenterol. 2012;18(20):2462-2471.
doi pubmed pmc - Ibeziako P, Brahmbhatt K, Chapman A, De Souza C, Giles L, Gooden S, Latif F, et al. Developing a clinical pathway for somatic symptom and related disorders in pediatric hospital settings. Hosp Pediatr. 2019;9(3):147-155.
doi pubmed - Cohen JA, Bukstein O, Walter H, Benson SR, Chrisman A, Farchione TR, Hamilton J, et al. Practice parameter for the assessment and treatment of children and adolescents with posttraumatic stress disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(4):414-430.
pubmed - Kullgren KA, Klein EJ, Sturza J, Hutton D, Monroe K, Pardon A, Sroufe N, et al. Standardizing pediatric somatic symptom and related disorders care: clinical pathway reduces health care cost and use. Hosp Pediatr. 2020;10(10):867-876.
doi pubmed - Bolger A, Collins A, Michels M, Pruitt D. Characteristics and outcomes of children with conversion disorder admitted to a single inpatient rehabilitation unit, a retrospective study. PM R. 2018;10(9):910-916.
doi pubmed - Butz C, Iske C, Truba N, Trott K. Treatment of functional gait abnormality in a rehabilitation setting: emphasizing the physical interventions for treating the whole child. Innov Clin Neurosci. 2019;16(7-08):18-21.
pubmed pmc - Kim YN, Gray N, Jones A, Scher S, Kozlowska K. The role of physiotherapy in the management of functional neurological disorder in children and adolescents. Semin Pediatr Neurol. 2022;41:100947.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.
