| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website http://www.neurores.org |
Original Article
Volume 2, Number 4, August 2012, pages 127-133
Effects of a PDGFR-a Antagonist Imatinib on Blood-Brain Barrier Disruption in Focal Cerebral Ischemia in Younger and Older Rats
Oak Z. Chia, c, Jeremy Graysona, Sylviana Barsouma, Christine Huntera, Xia Liua, Harvey R. Weissb
aDepartment of Anesthesia, Robert Wood Johnson Medical School, University of Medicine and Dentistry of New Jersey, 125 Paterson Street, Suite 3100, New Brunswick, New Jersey 08901-1977, U.S.A
bDepartment of Physiology and Biophysics, Robert Wood Johnson Medical School, University of Medicine and Dentistry of New Jersey, 675 Hoes Lane West, Piscataway, NJ 08854, U.S.A
cCorresponding author: Oak Z. Chi, Department of Anesthesia, Robert Wood Johnson Medical School, University of Medicine and Dentistry of New Jersey, 125 Paterson Street, Suite 3100, New Brunswick, New Jersey 08901-1977, U.S.A
Manuscript accepted for publication August 8, 2012
Short title: Imatinib on BBB in Focal Ischemia
doi: https://doi.org/10.4021/jnr118w
| Abstract | ▴Top |
Background: One of the pathways involving blood-brain barrier (BBB) disruption in focal ischemia can act through the platelet derived growth factor a receptor (PDGFR-a) system including PDGF-CC and tissue plasminogen activator (tPA). This study was performed to compare the effects of imatinib, a PDGFR-a antagonist, on blood-brain barrier (BBB) permeability in younger and older rats in focal cerebral ischemia and to determine whether the protein levels of PDGFR-a vary with age.
Methods: Three month old (Younger) and 24 month old (Older) male Fischer 344 rats were used. One hour before middle cerebral artery (MCA) occlusion, rats were treated by gavage with normal saline (control) or imatinib mesylate 200 mg/kg. At one and a half hours after MCAocclusion, BBB permeability was determined by measuring the transfer coefficient (Ki) of 14C-a-aminoisobutyric acid and volume of dextran distribution. Western blot analysis of PDGFR-a was performed on additional rats.
Results: With MCA occlusion, the Ki of the ischemic cortex (IC) significantly increased in both age groups. The Ki of the IC of the older control rats was significantly lower (-57%) than that of the younger control rats. In the younger rats, imatinib significantly decreased the Ki of the IC (-48%). In the older rats, however, imatinib failed to decrease the Ki. The difference in volume of dextran distribution between the IC and the contralateral cortex was significant only in the younger control rats and became insignificant with imatinib. Imatinib did not affect the volume of dextran distribution in the older rats. Neither aging nor imatinib affected BBB permeability in non-ischemic brain regions. The ratio of density of PDGFR-a normalized for tubulin level was similar between the younger and older rats (younger: 0.85 ± 0.48 vs older: 0.93 ± 0.58).
Conclusion: Imatinib decreased BBB disruption caused by focal cerebral ischemia in the younger rats but failed to decrease it in the older rats in spite of similar protein level of PDGFR-a.
Keywords: Aging; Blood-brain barrier permeability; Focal cerebral ischemia; Imatinib; PDGFR-a
| Introduction | ▴Top |
Disruption of the blood-brain barrier (BBB) in focal cerebral ischemia may cause vasogenic and cytotoxic edema and hemorrhage. One of the most important factors affecting BBB permeability in stroke could be aging. Aging may be associated with morphological and functional changes in BBB [1-3]. In focal cerebral ischemia, BBB permeability decreased with aging accompanied with decreased edema as well as infarction in male mice. In female mice, however, aging increased BBB permeability correlated with size of infarction [4]. In contrast, even in male animals, an increase in the size of infarction has been reported with aging [5, 6]. Still, the relationship of aging with BBB permeability and size of infarction is not clear.
One of the pathways involving BBB disruption in focal ischemia can act through the platelet derived growth factor a receptor (PDGFR-a) system including PDGF-CC and tissue plasminogen activator (tPA) [7]. Su et al reported that treatment of mice with a PDGFR-a antagonist imatinib after ischemic stroke reduced cerebrovascular permeability and stroke volume in the ischemic hemisphere. Imatinib also decreased hemorrhagic complication associated with late administration of thrombolytic tissue plasminogen activator (tPA) [7]. The PDGFR-a signaling system could be also altered with aging. With aging, alterations in PDGFs and PFGFRs in the brain and alterations in PDGFR-a signaling system in the vasculature have been reported [8-10].
The study on the effects of imatinib on BBB in cerebral ischemia was performed in young male animals [7]. Comparison between old and young animals has not been done. Since BBB disruption after MCA occlusion varied depending on age and sex of experimental animals and age may alter PDGFR-a system, this study was performed to test whether effects of imatinib on BBB disruption after MCA occlusion would vary depending on age in male rats. MCA occlusion was used to produce disruption of the BBB. The transfer coefficient (Ki) of 14C-a-aminoisobutyric acid (14C-AIB) across the BBB and the distribution of the volume of 3H-dextran in the brain of the older and younger male rats were determined to reflect the level of BBB disruption. Western blot analysis of PDGFR-a was determined in both ages.
| Materials and Methods | ▴Top |
Experimental animals and MCA occlusion
All experiments were approved by our Institutional Animal Care and Use Committee and were conducted in accordance with the Guide for the Care of Laboratory Animals (DHHS Publication No. 85-23, revised 1996). Three month old (Younger, n = 14) and 24 month old (Older, n = 14) male Fischer 344 rats were used in this study. They were anesthetized with isoflurane 2% in an air and oxygen mixture (inspired oxygen fraction 0.25 - 0.3), and were mechanically ventilated. A femoral artery and vein were catheterized and the femoral arterial catheter was connected to a Statham P23Db transducer (Gould Instruments, Cleveland, OH). Blood pressure was continuously monitored and recorded on a Beckman R-611 recorder (Fullerton, CA). A femoral venous catheter was used to administer medications and radioactive tracers.
One hour before a permanent middle cerebral artery (MCA) occlusion, half of each group of the rats was treated by gavage with 1 mL of normal saline (Younger or Older Control group) and the other half, with imatinib mesylate 200 mg/kg in 1 mL of normal saline (Younger or Older Imatinib treated group).
In order to expose the MCA, an incision was made at a midpoint between the right ear and the right eye. The temporalis muscle was separated and retracted to expose the squamosal bone and zygoma. The MCA was exposed through a hole about 3 mm in diameter near the anterior junction of the squamosal and the zygomatic bones. The dura and arachnoid membrane were carefully removed to expose the cerebral cortex. The MCA was occluded at the base with thermocoagulation using an electrocautery. After the craniotomy, the concentration of isoflurane was decreased to 1.4%.
Body temperature was maintained at 37 °C with a heating lamp and a servo-controlled rectal thermometer probe. Pericranial temperature was monitored using a thermocouple probe and maintained at approximately 37 °C. Prior to measuring the transfer coefficient, arterial blood pressure was recorded and a 0.2 mL arterial blood sample was drawn anaerobically and analyzed for PaO2, PaCO2, and pH, using a blood gas analyzer (ABL330, Radiometer America, Westlake, OH).
Determination of Ki
At one and a half hour after MCA occlusion, to determine the blood-brain transfer coefficient (Ki), 20 µCi of 14C-AIB (Amersham, Arlington Heights, Illinois) was rapidly injected intravenously and then was flushed with 0.5 mL of normal saline. Blood samples were collected from the arterial catheter at 20 sec intervals for the first 2 min and then, every min for the next 8 min. Five min after injecting 14C-AIB, 20 µCi of 3H-dextran (70,000 Da, Amersham, Arlington Heights, Illinois) was injected intravenously and flushed with 0.5 mL of normal saline. After collecting the ten min arterial blood sample, the animals were decapitated and their brains were quickly frozen in liquid nitrogen. The following brain regions were dissected: ischemic cortex (IC), contralateral cortex (CC) and pons. They were solubilized in Soluene before counting the radioactivity. Arterial blood samples were centrifuged and the plasma was separated. The plasma and the brain samples were processed in the same way for scintillation counting on a liquid scintillation counter that was equipped for dual label counting. Quench curves were prepared using carbon tetrachloride and all samples were automatically corrected for quenching. The blood-to-tissue transfer coefficient for 14C-AIB was determined, assuming a unidirectional transfer of 14C-AIB over a 10 min period of the experiment using the following equation as described by Gross et al [11] (Fig. 1): where Am is the equal amount of 14C-AIB radioactivity in the tissue per gram, Vp is the volume of plasma retained in the tissue determined from the 3H-dextran data, where Vp is obtained by dividing the amount of 3H-dextran radioactivity in the tissue per gram by the concentration of 3H-dextran in the plasma at the time of decapitation, Cp(t) is the arterial concentration of 14C-AIB over time t, and CT is the arterial plasma concentration of 14C-AIB at the time of decapitation. In the equation to determine Ki, Vp x CT is a correction term which accounts for the label 14C retained in the vascular compartment of the tissue, Am.
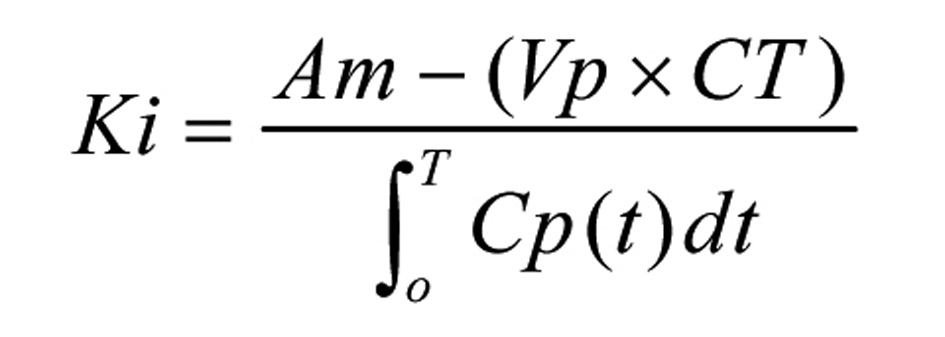 Click for large image | Figure 1. The blood-to-tissue transfer coefficient equation. |
Western Blot analysis of PDGFR-a
In three additional animals in each group, Western blot analysis of PDGFR-a was performed. Three cortical samples from each animal were homogenized in RIPA butter (Boston Bioproducts) containing a protease cocktail inhibitor (Roche). Using the BioRad protein assay (BioRad), protein concentrations were determined. Using the Mini-Protean blotting system (BioRad), proteins were separated with 8% SDS-PAGE, and then were transferred to PDVF membranes, which were soaked for 15 minutes in blotting buffer (Boston Bioproducts) containing 5% non-fat dry milk. They were then incubated overnight at 4 °C with either anti-PDGFR-a (Millipore) or anti-beta Tubulin (Abcam), then were washed in PBS. Membranes were then incubated with an anti-Rabbit-HRP conjugate (Millipore) in blotting buffer with 5% non-fat dry milk for 3 hours for PDGFR-a and two hours for Tubulin at room temperature. After washing in PBS the blots were enhanced and developed with HRP-based Oxidizing and Luminol solutions (Boston Bioproducts). Determination of the density of PDGFR-a was normalized by comparison with beta Tubulin internal standard. These are arbitrary values related to Tubulin.
Statistical analysis
A multifactorial analysis of variance was used to assess the differences among the brain regions and between the groups for various measurements performed. Analysis was conducted using the general linear model (PROC GLM) from the SAS Institute (Cary, NC). When differences were observed by a multifactorial analysis of variance, multiple comparisons were performed using the Tukey test. Differences were considered significant at P < 0.05 after correction for multiple comparisons. Statistical analysis of Western blot was performed using an unpaired t-test. All data were expressed as means ± SD.
| Results | ▴Top |
Hemodynamic and blood gas parameters were within the normal range for anesthetized rats in all the experimental groups. There were no significant differences among the experimental groups. Arterial blood gases, hemoglobin and pH were also not significantly different among all the experimental groups (Table 1).
 Click to view | Table 1. Hemodynamic Parameters for Experimental Groups One and a Half Hours After Middle Cerebral Artery Occlusion |
The effect of imatinib in the older and in the younger Fischer 344 rats on Ki during MCA occlusion is shown in Figure 2. MCA occlusion significantly increased Ki in all the experimental groups. In the younger animals, the Ki of the IC became significantly lower (-48%), P < 0.05) with imatinib administration. The Ki of the IC of the Older Control rats was significantly lower (-57%, P < 0.05) than that of the Younger Control rats. In the older rats, imatinib did not lower the Ki. The Ki of the CC was similar among the experimental groups. The Ki values in the pons were not significantly different among the four experimental groups and were similar to those of the corresponding CC (Younger Control: 2.72 ± 1.90, Younger Imatinib: 2.07 ± 0.95, Older Control: 2.10 ± 0.45, Older Imatinib: 2.48 ± 0.79 mL/g/min).
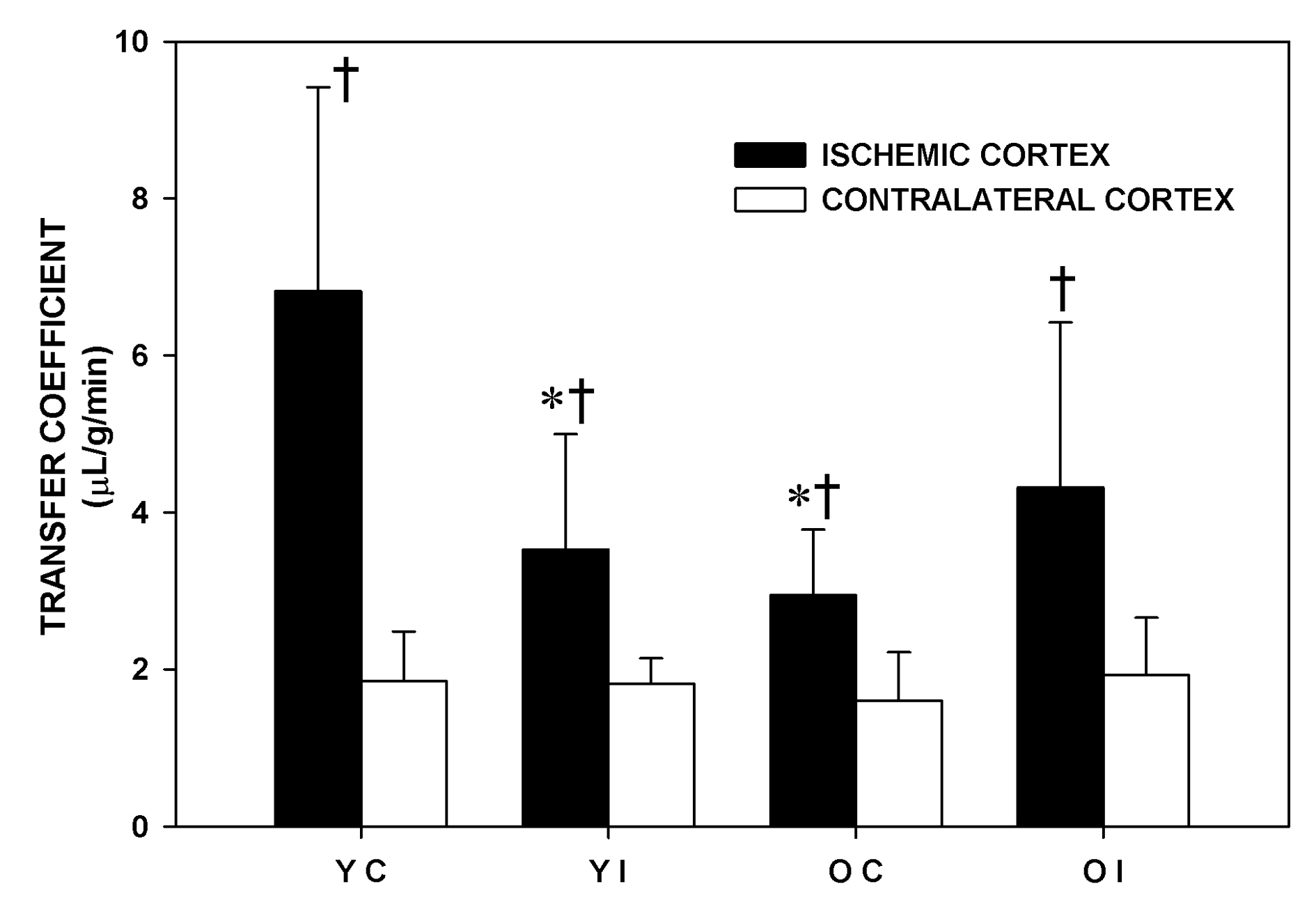 Click for large image | Figure 2. Transfer coefficient (Ki) of the experimental groups one and a half hour after middle cerebral artery occlusion. YC: younger control rats. YI: younger imatinib treated rats. OC: older control rats. OI: older imatinib treated rats. IC: Ischemic cortex. CC: Contralateral cortex. Data are Means ± SD. * Significantly different from the YC. † Significantly different from the corresponding CC. |
The volume of dextran distribution represents the intravascular volume and the volume of the dextran extravasated due to BBB disruption. The effects of imatinib on the volume of dextran distribution are shown in Figure 3. The difference in the volume of dextran distribution between the IC and the CC was significant only in the Younger Control group, not in other experimental groups. The difference became insignificant with imatinib administration in the Younger rats. Imatinib did not affect the volume of dextran distribution in the older rats in any of the brain regions that were studied. There were no significant differences in the volume of dextran distribution of the IC and CC among the experimental groups. The volume of dextran distribution of the pons was similar among the experimental groups and similar to that of the corresponding contralateral cortex (Younger Control: 3.30 ± 1.15, Younger Imatinib: 2.98 ±1.08, Older Control: 3.20 ± 0.53, Older Imatinib: 3.05 ± 0.65 mL/100 g respectively).
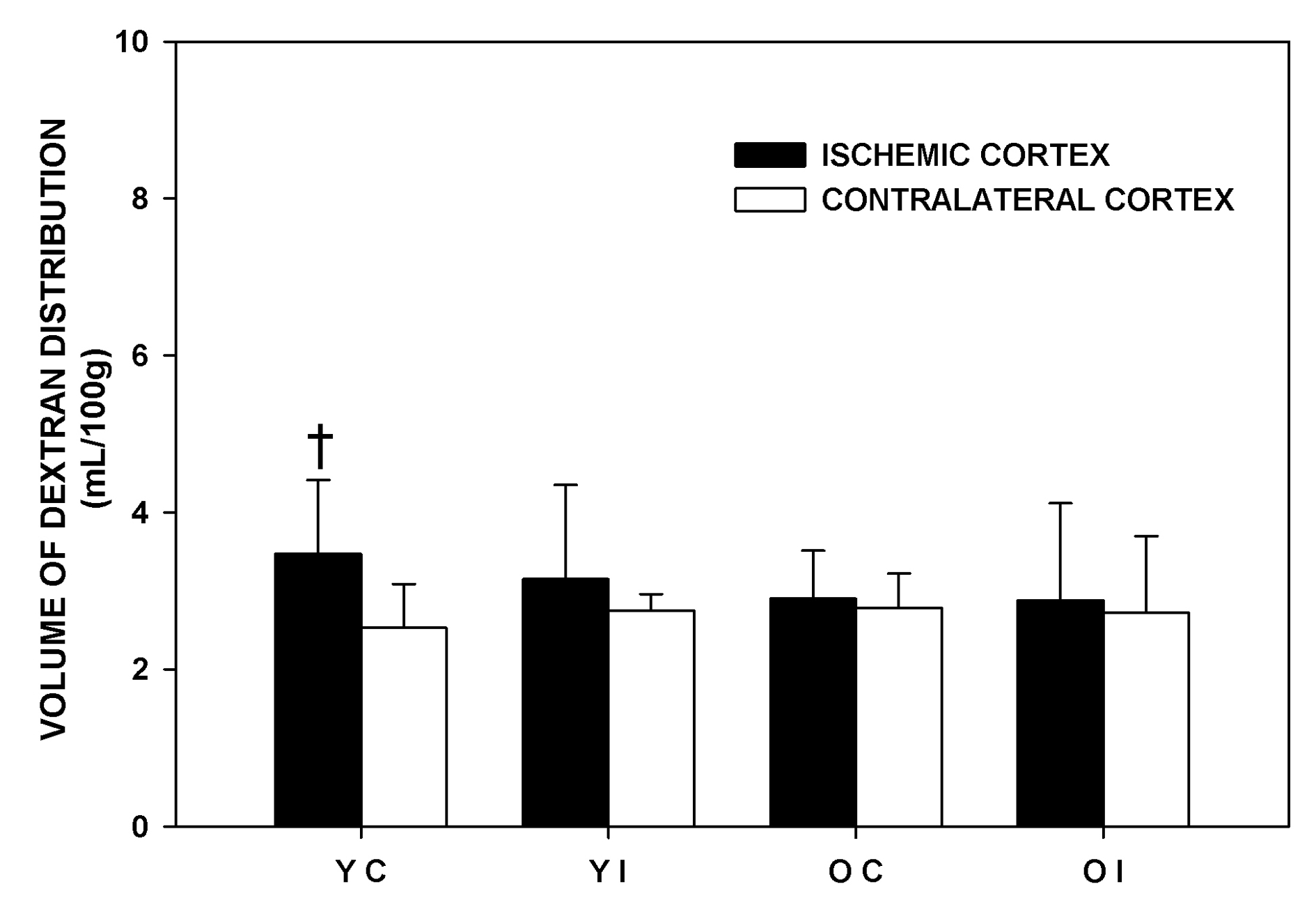 Click for large image | Figure 3. Volume of dextran distribution of the experimental groups one and a half hours after middle cerebral artery occlusion. YC: younger control rats. YI: younger imatinib treated rats. OC: older control rats. OI: older imatinib treated rats. IC: Ischemic cortex. CC: Contralateral cortex. Data are Means ± SD. †Significantly different from the corresponding CC. |
The ratio of density of PDGFR-a normalized for tubulin level was similar between the younger and the older rats (younger: 0.85 ± 0.48 vs older: 0.93 ± 0.58). A representative Western blot is shown in the Figure 4.
 Click for large image | Figure 4. Protein levels of the PDGRF-asubunit probed with a specific antibody measured by Western blotting. Tubulin was used as the control. The cortices from three young (Y) and three old (O) Fischer 344 rats are shown. The protein levels of the PDGFR-a subunit were similar between the younger and the older rats. |
| Discussion | ▴Top |
Our main finding was that the BBB disruption in focal ischemia was attenuated with imatinib pretreatment only in the Younger but not in the Older rats. The BBB disruption by MCA occlusion was less in the Older than in the Younger rats. Neither aging nor imatinib affected BBB permeability in non-ischemic brain regions. Aging did not affect protein level of PDGFR-a. Our data suggest that the role of imatinib in protecting the BBB changes with aging.
MCA occlusion is associated with BBB disruption, the degree of which could vary with many factors. The method of MCA occlusion, the size of the tracer molecules, reperfusion, blood pressure, gender, age, species and anesthetic agents may all influence the disruption. We used two kinds of molecules of different sizes to determine the BBB disruption; 14C-AIB is a small synthetic inert hydrophilic neutral amino acid whose molecular weight is 104 Daltons while 3H-dextran is a large molecule with molecular weight of 70,000 Daltons. At a given degree of BBB disruption, smaller molecules may pass through the BBB but not the larger ones. Our data showed that in all groups of rats the Ki was significantly higher in the ischemic than the contralateral cortex. However, the volume of dextran distribution was significantly higher in the ischemic than the contralateral cortex only in the younger control rats and the degree of difference between the cortices was much smaller than that of Ki. These data suggest that there is some degree of BBB disruption after MCA occlusion that allows small molecules to pass through easily but not large ones. The volume of dextran distribution represents the volume inside the vessel as well as extravasated volume. Therefore it could reflect the degree of BBB disruption. The volume of dextran distribution is also a correction term for obtaining Ki. Transfer of a substrate across the BBB is dependent on the product of permeability itself (P) and perfused capillary area (S). Since the PS product of 14C-AIB was extremely low and almost identical to the value of Ki in the same experimental model, the Ki of 14C-AIB should represent the degree of disruption of the BBB [12, 13].
Aging may be one of the factors which affect BBB permeability in the ischemic and non-ischemic brain tissue by affecting perfused capillary area and/or permeability itself. There are reports that in Fischer 344 rats the permeability-surface area product (PS) of 3H or 14C-sucrose across the BBB was not altered with aging [14]. Perfused capillary morphometry did not significantly change with increasing age [15]. With aging there are some histological changes in the BBB but its functional aspects may not be altered [2, 14]. These data are similar to ours from non-ischemic area. However, there are other studies where PS product is decreased for tryptophan in older Sprague-Dawley rats [3]. Differences in BBB disruption and the size of infarction after MCA occlusion depending on age and sex have been reported. Liu et al reported that the BBB permeability after MCA occlusion measured with Evans blue decreased with aging in male mice but increased in the old female mice when compared with younger mice [4]. Edema was significantly less in aged mice in both sexes. BBB permeability changes were correlated with the size of infarction. The decreased BBB disruption in the focal ischemic area observed in the Older Control male rats in this study is similar to Liu et al’s. To the contrary, an age related increase in the cerebral infarct size in male rats has been reported [5, 6]. The effects of aging on BBB disruption and the size of infarction in focal cerebral ischemia are not settled.
Changes involved in tPA/PDGFCC/PDGFR-a signaling pathways with aging could have prevented the attenuating effects of imatinib on BBB permeability in focal cerebral ischemia in older brain. Aging was associated with a selective lowering of brain tPA expression [16]. The levels of PDGFR-a, PDGFR-b and PDGF, and phosphorylation in the brain decreased with aging [8]. However, in the vasculature, expression of mRNA of PDGFR-a and PDGFR-a was increased with aging [9, 10]. It takes time to induce a significant elevation of PDGFR-a protein after cerebral hypoxia or ischemia [7, 17]. The younger and the older animals had a similar protein level of PDGFR-a in non-ischemic brain in our study. These studies suggest that other factors affected by aging beside the PDGFR-a system could have also contributed to this lack of effects of imatinib on BBB disruption in the older rats. Further exploration is needed to clarify the role of PDGFR-a in BBB disruption in aged brain.
The tyrosine kinase inhibitor, imatinib is known as PDGFR-a antagonist and may alter various signaling pathways. Imatinib inhibits autophosphorylation of various receptors or factors such as PDGFR-a, b, C kit and BCR-ABL and has proven successful in treatment of chronic myeloid leukemia [18]. The signaling pathways of tPA/PDGFCC/PDGFR-a have been implicated in BBB disruption in cerebral ischemia [8]. It has been shown that tPA cleaves and activates PDGF-CC which became PDGFR-a agonist [7, 19, 20]. Using ten week old mice, Su et al demonstrated that administration of imatinib, a PDGFR-a antagonist, after MCA occlusion reduced cerebrovascular permeability, a 33% reduction in Evans blue extravasation compared with the control. Imatinib significantly decreased infarct volume by 34% at 72 hours after MCA occlusion. Imatinib also reduced hemorrhagic complication by 50 % associated with the late treatment of tPA [7]. Our data which demonstrated 48% reduction in Ki in the ischemic cortex in the Younger rats with imatinib treatment are similar to their study.
Since imatinib is involved in various signaling pathways, the attenuation of BBB disruption induced by imatinib in the young animals could be due to other factors beside PDGFR-asignaling pathways. PDGF-B/PDGFR-b signaling which is also a target receptor of imatinib, is necessary for pericyte recruitments during angiogenesis [18, 21] and the critical role of pericytes is regulating the BBB [22, 23]. The loss of pericytes in the elderly has been reported suggesting less compensation for transient leakage of BBB [24]. PDGFR-b was also reported to decrease with aging [8]. Thus, reduced effects of imatinib in old animals may be related to age induced changes in PDGFR-a signaling or changes in other signaling pathways affected by imatinib. It is possible that specific proteins of tight junctions such as claudin and occludin may be altered with aging [1, 25]. Thus, ischemia in old age may cause BBB disruption through altered mechanisms which may not be affected with imatinib treatment. Therefore, the beneficial effects of imatinib on the BBB may be obtained only in the younger animals.
In conclusion, our data demonstrated that the BBB disruption was greater in the younger rats when compared with older ones in the early stages of stroke. Imatinib attenuated BBB disruption in the younger rats but not in the older rats in spite of similar protein level of PDGFR-a. Our data suggest that imatinib exerts different effects on BBB in focal cerebral ischemia depending on age. For clinical application of imatinib in stroke, more studies are needed to clear its different effects in young and old animals.
Financial Disclosures
Oak Z. Chi: No financial arrangement to disclose; Jeremy Grayson: No financial arrangement to disclose; Sylviana Barsoum: No financial arrangement to disclose; Christine Hunter: No financial arrangement to disclose; Xia Liu: No financial arrangement to disclose; Harvey R. Weiss: No financial arrangement to disclose.
| References | ▴Top |
- Kaur J, Tuor UI, Zhao Z, Barber PA. Quantitative MRI reveals the elderly ischemic brain is susceptible to increased early blood-brain barrier permeability following tissue plasminogen activator related to claudin 5 and occludin disassembly. J Cereb Blood Flow Metab. 2011;31(9):1874-1885.
pubmed doi - Mooradian AD. Effect of aging on the blood-brain barrier. Neurobiol Aging. 1988;9(1):31-39.
pubmed doi - Tang JP, Melethil S. Effect of aging on the kinetics of blood-brain barrier uptake of tryptophan in rats. Pharm Res. 1995;12(7):1085-1091.
pubmed doi - Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab. 2009;29(4):792-802.
pubmed doi - Davis M, Mendelow AD, Perry RH, Chambers IR, James OF. Experimental stroke and neuroprotection in the aging rat brain. Stroke. 1995;26(6):1072-1078.
pubmed doi - Kharlamov A, Kharlamov E, Armstrong DM. Age-dependent increase in infarct volume following photochemically induced cerebral infarction: putative role of astroglia. J Gerontol A Biol Sci Med Sci. 2000;55(3):B135-141; discussion B142-133.
pubmed - Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J, Gao Y, et al. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med. 2008;14(7):731-737.
pubmed doi - Hu Y, Schett G, Zou Y, Dietrich H, Xu Q. Abundance of platelet-derived growth factors (PDGFs), PDGF receptors and activation of mitogen-activated protein kinases in brain decline with age. Brain Res Mol Brain Res. 1998;53(1-2):252-259.
pubmed doi - Vazquez-Padron RI, Lasko D, Li S, Louis L, Pestana IA, Pang M, Liotta C, et al. Aging exacerbates neointimal formation, and increases proliferation and reduces susceptibility to apoptosis of vascular smooth muscle cells in mice. J Vasc Surg. 2004;40(6):1199-1207.
pubmed doi - Yang XP, Pei ZH, Ren J. Making up or breaking up: the tortuous role of platelet-derived growth factor in vascular ageing. Clin Exp Pharmacol Physiol. 2009;36(8):739-747.
pubmed doi - Gross PM, Blasberg RG, Fenstermacher JD, Patlak CS. The microcirculation of rat circumventricular organs and pituitary gland. Brain Res Bull. 1987;18(1):73-85.
pubmed doi - Chi OZ, Anwar M, Sinha AK, Wei HM, Klein SL, Weiss HR. Effects of isoflurane on transport across the blood-brain barrier. Anesthesiology. 1992;76(3):426-431.
pubmed doi - Chi OZ, Wei HM, Anwar M, Sinha AK, Klein SL, Weiss HR. Effects of fentanyl on alpha-aminoisobutyric acid transfer across the blood-brain barrier. Anesth Analg. 1992;75(1):31-36.
pubmed doi - Wadhwani KC, Koistinaho J, Balbo A, Rapoport SI. Blood-nerve and blood-brain barrier permeabilities and nerve vascular space in Fischer-344 rats of different ages. Mech Ageing Dev. 1991;58(2-3):177-190.
pubmed doi - Buchweitz-Milton E, Weiss HR. Perfused capillary morphometry in the senescent brain. Neurobiol Aging. 1987;8(3):271-276.
pubmed doi - Roussel BD, Macrez R, Jullienne A, Agin V, Maubert E, Dauphinot L, Potier MC, et al. Age and albumin D site-binding protein control tissue plasminogen activator levels: neurotoxic impact. Brain. 2009;132(Pt 8):2219-2230.
pubmed doi - Morioka I, Tsuneishi S, Takada S, Matsuo M. PDGF-alpha receptor expression following hypoxic-ischemic injury in the neonatal rat brain. Kobe J Med Sci. 2004;50(1-2):21-30.
pubmed - Capdeville R, Buchdunger E, Zimmermann J, Matter A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat Rev Drug Discov. 2002;1(7):493-502.
pubmed doi - Fredriksson L, Ehnman M, Fieber C, Eriksson U. Structural requirements for activation of latent platelet-derived growth factor CC by tissue plasminogen activator. J Biol Chem. 2005;280(29):26856-26862.
pubmed doi - Li X, Ponten A, Aase K, Karlsson L, Abramsson A, Uutela M, Backstrom G, et al. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol. 2000;2(5):302-309.
pubmed doi - Winkler EA, Bell RD, Zlokovic BV. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol Neurodegener. 2010;5:32.
pubmed - Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468(7323):557-561.
pubmed doi - Dalkara T, Gursoy-Ozdemir Y, Yemisci M. Brain microvascular pericytes in health and disease. Acta Neuropathol. 2011;122(1):1-9.
pubmed doi - Stewart PA, Magliocco M, Hayakawa K, Farrell CL, Del Maestro RF, Girvin J, Kaufmann JC, et al. A quantitative analysis of blood-brain barrier ultrastructure in the aging human. Microvasc Res. 1987;33(2):270-282.
pubmed doi - Mooradian AD, Haas MJ, Chehade JM. Age-related changes in rat cerebral occludin and zonula occludens-1 (ZO-1). Mech Ageing Dev. 2003;124(2):143-146.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.
