| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website http://www.neurores.org |
Case Report
Volume 3, Number 5, October 2013, pages 155-158
Optic Nerve Radiation Recall Associated With Prior Pemetrexed
Rimas V. Lukasa, e, Jacqueline T. Bernarda, Renuka Malikb, Ashley Finchc, Ravi Salgiad
aDepartment of Neurology, University of Chicago, Chicago, USA
bDepartment of Radiation and Cellular Oncology, University of Chicago, Chicago, USA
cIllinois College of Optometry, Chicago, USA
dSection of Hematology and Oncology, University of Chicago, Chicago, USA
eCorresponding author: Rimas V. Lukas, 5841 South Maryland Avenue, MC 2030 Chicago, Illinois 60637, USA
Manuscript accepted for publication October 15, 2013
Short title: Optic Nerve RT Recall
doi: https://doi.org/10.4021/jnr239e
| Abstract | ▴Top |
Radiation recall is an inflammatory response which presents in previously radiated tissue after administration of chemotherapy. We present a case of radiation recall involving the bilateral optic nerves after administration of pemetrexed in a patient who received prior whole brain radiation therapy. Optical coherence tomography imaging revealed significant thinning of the retinal nerve fiber layer. This is the first case of central nervous system radiation recall reported in association with pemetrexed. These are also the first published optical coherence tomography images of radiation recall optic neuropathy.
Keywords: Pemetrexed; Radiation recall reaction; Optic neuropathy; Optical coherence tomography; Non-small cell lung cancer
| Introduction | ▴Top |
Radiation recall is a rare but well described phenomenon in which an inflammatory response develops in previously radiated tissue during subsequent administration of chemotherapy. Although most frequently noted as a cutaneous reaction, it has also been described occurring in pulmonary tissue and the central nervous system (CNS).[1] Radiation recall has been described in association with various classes of chemotherapeutic agents including with antimetabolites such as the folic acid metabolism inhibitor, methotrexate [2]. Non-CNS radiation recall has been described with carboplatin in combination with paclitaxel as well as with erlotinib [3-5]. While radiation recall dermatitis has been reported in association with pemetrexed, another folic acid inhibiting antimetabolite, to our knowledge, there are no reports of pemetrexed associated CNS radiation recall or optic neuropathy.
Direct CNS toxicity, including optic neuropathy, has been described with numerous chemotherapeutic agents. Necrosis of the optic nerve has occurred in association with carboplatin after periocular or intracarotid injections [6, 7]. Development of optic neuritis has also been described 13 weeks after the completion of therapy with intravenous carboplatin plus cisplatin [8]. CNS toxicity with the use of methotrexate after whole brain radiation therapy (WBRT) has long been known [9]. This primarily manifests as a diffuse leukoencephalopathy, particularly in older patients. There have been reports of posterior optic neuropathy in association with methotrexate alone without radiation [10]. It is thought that homocysteine mediated vascular injury may be a potential mechanism by which this CNS injury occurs [11]. CNS, including optic nerve, toxicity has not been described in association with erlotinib and has not been previously reported with pemetrexed.
| Case Report | ▴Top |
We present a 61-year-old female diagnosed with EGFR mutated non-small cell lung cancer and synchronous brain metastases. She received WBRT to 35 Gy in 2.5 Gy fractions as well as 50 Gy to the primary tumor in the lung and mediastinum and 30 Gy to a symptomatic bone metastasis in the hip. She was then initiated on carboplatin (AUC 6) and pemetrexed (500 mg/m2) on 21-day cycles with concomitant erlotinib (150 mg daily). She received a total of 5 cycles of carboplatin and pemetrexed and continued on maintenance erlotinib. During the course of her treatment, she developed a deep venous thrombosis for which she was initiated on low-molecular weight heparin. Approximately 8 months after the completion of WBRT, she developed the sudden onset of right sided sensory symptomatology and aphasia which resolved in less than 24 h while her low-molecular weight heparin was temporarily on hold. Work-up for transient ischemic attack included an unremarkable echocardiogram and low-molecular weight heparin was resumed. Approximately 2 weeks after this event, she developed the subacute onset of left eye decrease in vision. It began initially in the upper temporal quadrant and progressed to involve the entire visual field in the left eye. Vision was 20/200 pinholing to 20/80 in the left eye and 20/40 in the right. There was no evidence of papilledema, optic nerve atrophy, or ischemic injury. Brain MRI revealed a new area of enhancement in the posterior left optic nerve (Fig. 1A, B). There was concern for a new CNS metastasis and the patient urgently initiated focal radiation to the area of enhancement under the care of her local physicians.
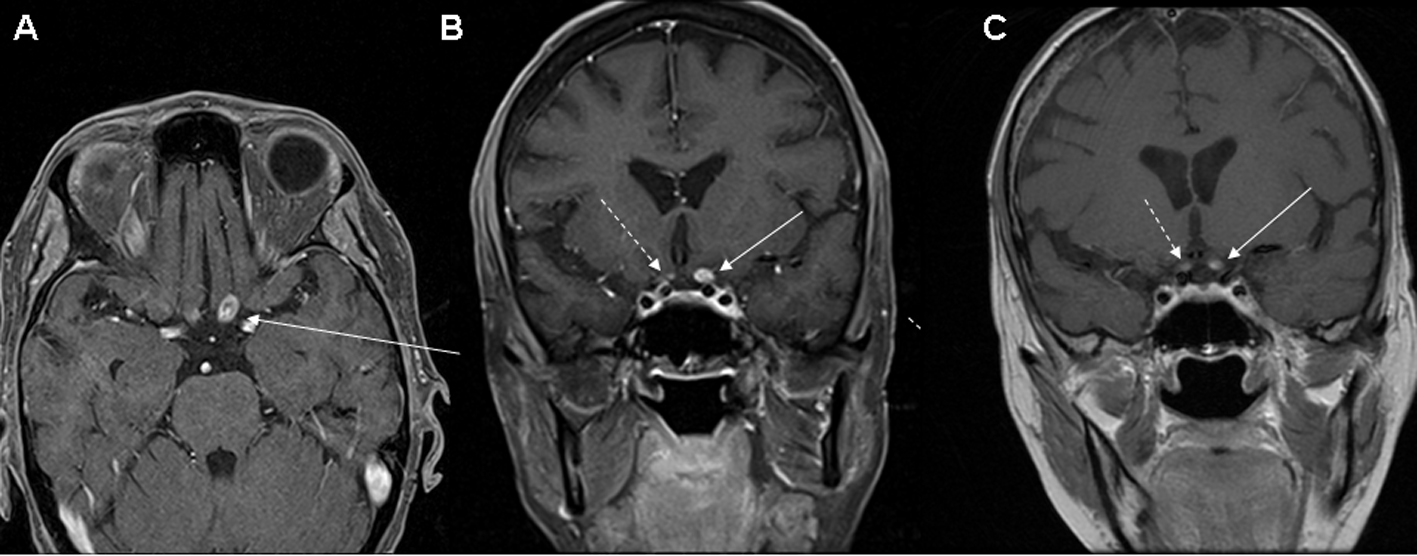 Click for large image | Figure 1. (A) Axial, and (B, C) coronal T1 post-contrast MRI images revealing an enhancing lesion of the posterior portion of the left optic nerve (solid arrow). There is also a smaller area of possible enhancement in the right optic nerve (dashed arrow) which is more clearly defined on the MRI (C) performed 6 weeks after the earlier study (B). |
Our initial evaluation of the patient during the course of her focal radiation revealed a neurological examination only remarkable for markedly decreased vision in the left eye and an afferent pupillary defect. Serum studies evaluating for metabolic (B1 149 nmol/L, B12 510 pg/mL), autoimmune (aquaporin-4, ANNA-1, ANNA-2, ANNA-3, PCA1, PCA2, PCA Tr, amphiphysin, CRMP 5 IgG, striational, P/Q type calcium channel, N type calcium channel, AchR binding, AChR ganglionic, VGKC, and anti-glial nuclear type 1 antibodies) etiologies were unremarkable. CT angiogram of the head and neck revealed no carotid stenosis. Visual evoked potentials (VEP) revealed no responses with the left eye and mildly delayed response in the asymptomatic right eye indicating subclinical deficit. Optical coherence tomography (OCT) demonstrated macular sparing with bilateral asymmetric thinning of the retinal nerve fiber layer (RNFL)(Fig. 2). The patient was treated with an empiric course of steroids without significant clinical improvement. Follow-up MRI performed 6 weeks after the initial study revealed continued presence of the left optic nerve enhancement with more obvious right optic nerve enhancement. The enhancement appeared to be intrinsic to the optic nerves and not consistent with compressive lesions (Fig. 1C).
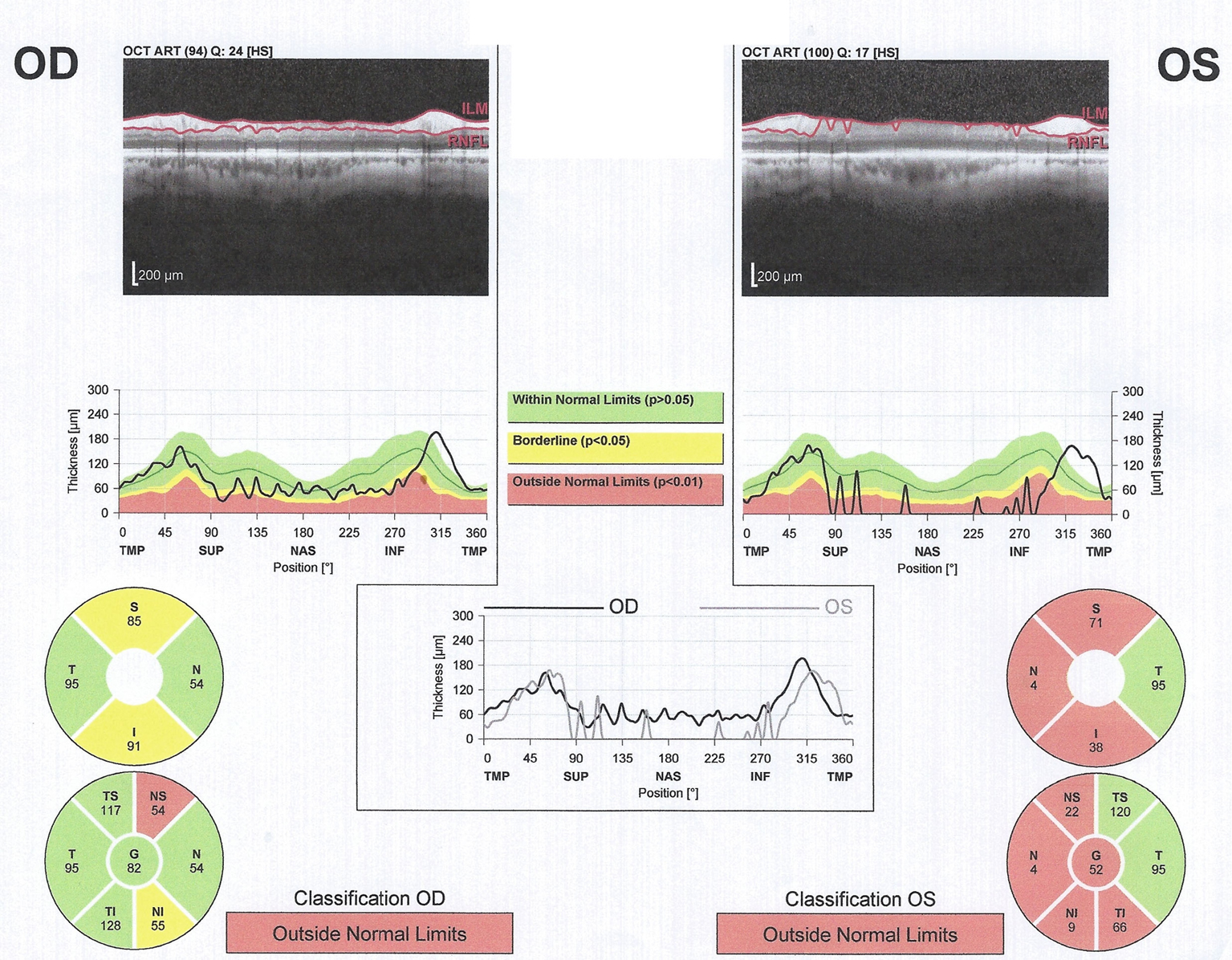 Click for large image | Figure 2. Optical coherence tomography. Left (OS) greater than right (OD) retinal nerve fiber layer (RNFL) thinning is demonstrated.” In the current iteration the Left and Right are switched. |
| Discussion | ▴Top |
We present a report of optic neuropathy representing a CNS manifestation of radiation recall related to pemetrexed or to a combination of pemetrexed with carboplatin and erlotinib. Additionally, we present the first use of OCT in the evaluation of CNS radiation recall. Alternative explanations for her symptomatology include carboplatin or an ischemic event. With carboplatin, the previously described optic neuropathy occurred over a much shorter interval after systemic treatment or was the result of direct periocular injection. The patient’s subacute time course of symptomatology, posterior optic nerve location of bilateral enhancement of the optic nerves on MRI, lack of vascular imaging abnormalities, delayed VEP bilaterally, and bilateral RNFL thinning on OCT make this less likely. These OCT findings typically require 3-6 months to develop, raising suspicion that the process began prior to development of MRI abnormalities.
While radiation recall is not frequently encountered clinically, raised awareness may assist in its identification and management. In our case, we postulate whether a similar pathophysiology underlying chronic leukoencephalopathy and optic neuropathy associated with methotrexate may have contributed to our patient’s symptoms. Unlike with methotrexate associated CNS toxicity after radiation, our patient had significant enhancement at the site of her greatest symptomatology. This enhancement is consistent with a robust inflammatory response as would be expected in radiation recall.
Conflicts of Interests Statement
The authors report no conflicts of interest.
Grant Support
NIH/National Cancer Institute (5R01CA125541-03, 3R01CA125541-03S109, 5R01CA129501-02, 3R01CA129501-02S109, and 5P01HL058064-140009), V-Foundation (Guy Gelded Memorial Foundation), Kate McMullen Foundation, Respiratory Health Association of Chicago, and Mesothelioma Applied Research Foundation (Jeffrey P. Hayes Memorial Grant) (RS).
| References | ▴Top |
- Jeter MD, Janne PA, Brooks S, Burstein HJ, Wen P, Fuchs CS, Loeffler JS, et al. Gemcitabine-induced radiation recall. Int J Radiat Oncol Biol Phys. 2002;53(2):394-400.
doi - Adrian RM, Hood AF, Skarin AT. Mucocutaneous reactions to antineoplastic agents. CA Cancer J Clin. 1980;30(3):143-157.
doi pubmed - Kundak I, Oztop I, Soyturk M, Ozcan MA, Yilmaz U, Meydan N, Gorken IB, et al. Paclitaxel-carboplatin induced radiation recall colitis. Tumori. 2004;90(2):256-258.
pubmed - Onal C, Abali H, Koc Z, Kara S. Radiation recall pneumonitis caused by erlotinib after palliative definitive radiotherapy. Onkologie. 2012;35(4):191-194.
doi pubmed - Dauendorffer JN, Dupuy A. Radiation recall dermatitis induced by erlotinib. J Am Acad Dermatol. 2009;61(6):1086.
doi pubmed - Schmack I, Hubbard GB, Kang SJ, Aaberg TM, Jr., Grossniklaus HE. Ischemic necrosis and atrophy of the optic nerve after periocular carboplatin injection for intraocular retinoblastoma. Am J Ophthalmol. 2006;142(2):310-315.
doi pubmed - Watanabe W, Kuwabara R, Nakahara T, Hamasaki O, Sakamoto I, Okada K, Minamoto A, et al. Severe ocular and orbital toxicity after intracarotid injection of carboplatin for recurrent glioblastomas. Graefes Arch Clin Exp Ophthalmol. 2002;240(12):1033-1035.
doi pubmed - Caraceni A, Martini C, Spatti G, Thomas A, Onofrj M. Recovering optic neuritis during systemic cisplatin and carboplatin chemotherapy. Acta Neurol Scand. 1997;96(4):260-261.
doi pubmed - DeAngelis LM, Yahalom J, Thaler HT, Kher U. Combined modality therapy for primary CNS lymphoma. J Clin Oncol. 1992;10(4):635-643.
pubmed - Sbeity ZH, Baydoun L, Schmidt S, Loeffler KU. Visual field changes in methotrexate therapy. Case report and review of the literature. J Med Liban. 2006;54(3):164-167.
pubmed - Mahadeo KM, Dhall G, Panigrahy A, Lastra C, Ettinger LJ. Subacute methotrexate neurotoxicity and cerebral venous sinus thrombosis in a 12-year-old with acute lymphoblastic leukemia and methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: homocysteine-mediated methotrexate neurotoxicity via direct endothelial injury. Pediatr Hematol Oncol. 2010;27(1):46-52.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.







