| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website http://www.neurores.org |
Case Report
Volume 2, Number 5, October 2012, pages 215-220
Endovascular Management of Cerebral Arteriovenous Malformations in Pregnancy: Two Case Reports and a Review of the Literature
Nitin Agarwala, Grant Schaletb, Manan Shaha, Peter Svidera, Charles J. Prestigiacomoa, c, d, Chirag D. Gandhia, c, e
aDepartment of Neurological Surgery, New Jersey Medical School, University of Medicine and Dentistry of New Jersey, Newark, New Jersey, USA
bDepartment of Biology, Washington University in St. Louis, The College of Arts and Sciences, St. Louis, Missouri, USA
cDepartment of Radiology, New Jersey Medical School, University of Medicine and Dentistry of New Jersey, Newark, New Jersey, USA
dDepartment of Neurology and Neuroscience, New Jersey Medical School, University of Medicine and Dentistry of New Jersey, Newark, New Jersey, USA
eCorresponding author: Chirag D. Gandhi, Neurosurgery TBI Research, Neurological Institute of New Jersey, New Jersey Medical School, 90 Bergen Street, Suite 8100 Newark, NJ 07101-1709, USA
Manuscript accepted for publication October 1, 2012
Short title: Endovascular Management
doi: https://doi.org/10.4021/jnr148w
| Abstract | ▴Top |
Cerebral arteriovenous malformations are rare, congenital lesions that affect the vasculature of the brain and have the possibility of rupturing. The presentation of a cerebral arteriovenous malformations in a pregnant woman warrants an even greater level of concern due to the maternal physiological changes, which may affect the structural integrity of the arteriovenous malformations and lead to hemorrhaging. A proper plan of action should be deployed to successfully treat pregnant women with cerebral arteriovenous malformations. In all cases, both the mother’s and fetus’s well being must be taken into consideration. Two cases and the current management options for cerebral arteriovenous malformations in pregnancy are presented. Online databases, such as PubMed provided by the United States National Library of Medicine at the National Institutes of Health, were searched for references to convey endovascular management options for cerebral arteriovenous malformations in pregnancy. Additional references were obtained from articles that were reviewed by the authors. The first case is of a 27-year-old pregnant woman with a right-sided periventricular intraparenchymal hemorrhage. She was treated with an external ventriculostomy drain. The second case is of a 26-year-old pregnant woman with a prior stage 2 embolization of a left parietal arteriovenous malformation. She did not show evidence of intracranial hemorrhage and was treated with further embolization regimens. A multifaceted approach, tailored to the individual patient may be necessary for cerebral arteriovenous malformations in pregnancy. Successful therapeutic strategies often involve close collaboration with a group of obstetricians, neuroradiologists, anesthesiologists, and neurosurgeons.
Keywords: Cerebral; Arteriovenous malformation; Pregnancy; Endovascular; Management
| Introduction | ▴Top |
An arteriovenous malformation (AVM) is a rare, congenital anomaly most commonly found in the brain, consisting of an abnormal connection between arteries and veins. This vascular lesion is prevalent in approximately 0.01% of the general population, affecting both men and women at about the same rate, and is quite severe with detrimental complications [1-5]. It has been stated that the development of an AVM might be the effect of one or several triggers on a gene defect of the post-capillary endothelium [6, 7]. Others have proposed the idea that an AVM might arise through a pathological hemodynamic response to exogenous factors, such as trauma [2]. AVMs typically develop in the third week of fetal development and are frequently asymptomatic [8]. AVMs account for 1-2% of all strokes, and about 53% present with a hemorrhage during their lifetime [1, 3]. Other clinical presentations include generalized or focal seizures (30% of patients) and persistent or progressive neurological deficit (12%) [3]. The modifications in maternal cardiovascular physiology that take place during pregnancy may impose a threat to the stability of the cerebral AVM [9]. It has been proposed that the increased cardiac output or circulatory effects of the elevated estrogen levels may be the link between AVM rupture and pregnancy [10]. These changes may increase the risk of hemorrhage in pregnant women and thus warrant an even greater level of concern. Moreover, rupture of an intracranial AVM during pregnancy requires immediate attention to the mother’s life as well as the fetus’ well being. Even though there is inconclusive data on AVMs during pregnancy, there is general agreement that the management of AVMs during pregnancy should primarily be based on neurological, rather than obstetric, considerations [11]. Historically, AVMs have been treated surgically with great success but with recent advances in endovascular technology, embolization has been used as an effective complement [12]. The authors present a review of the multimodal endovascular treatments for cerebral AVMs and two cases that portray the management dilemma that is encountered from cerebral arteriovenous malformations during pregnancy. Specific endovascular therapeutic strategies are addressed with consideration of vascular changes during pregnancy.
| Endovascular Treatment | ▴Top |
Treatment decision depends on numerous factors that allow for the lowest risk and greatest efficacy including the patient’s age, neurological condition, associated clinical risk factors, and features of the AVM lesions that are determined through imaging [3]. Endovascular embolization treatment is based on occluding blood flow to an AVM by depositing embolic materials into intracranial vessels using flow-directed and flow-assisted microcatheters [13]. The embolic materials currently in use are either solid or liquid agents. Solid agents include polyvinyl alcohol particles, fibers, microcoils, and microballoons. Some liquid agents include cyanoacrylate monomers such as IBCA (I-butyl cyanoacrylate) and NBCA (N-butyl cyanoacrylate), and polymer solutions such as ethylene vinyl alcohol (EVAL copolymer). Another liquid agent is absolute ethanol [13]. When used as the sole treatment, endovascular embolization has shown to be marginally efficacious, with success in only about 10% of patients [14-17]. It has been cited as being more effective when employed in the context of a multimodal approach to treating an AVM, shrinking its size of the AVM to improve its candidacy for other interventions [18, 19]. The use of endovascular embolization in a multimodal treatment approach can be divided into three categories: presurgical embolization, embolization before radiosurgery, and palliative embolization. In general, the literature shows that there are two major methods used when managing a patient undergoing endovascular embolization. The first utilizes general anesthesia during embolization, believing that knowledge of neuroanatomy and vascular architecture is sufficient for one to calculate the probability of neurological damage after the procedure. The second uses deep intravenous sedation, but allows for patient movement given that this will result in greater knowledge of the true functional anatomy of the patient since he or she would be responsive [13]. Nevertheless, each embolization procedure carries with it a calculated death rate of 1.2%, indicating that it is certainly not free of risk and should be employed with some caution [20, 21].
Presurgical embolization
Endovascular embolization prior to microsurgery is largely used when treating large AVM lesions [22-26]. Such a procedure has numerous advantages such as reduced blood loss, shorter surgical times, increased ability to occlude vessels difficult to control, and the benefit of staging flow reduction in the nidus [13, 18]. The primary goal of presurgical embolization is to decrease the nidus size of the AVM as well as to occlude deep, surgically inaccessible blood vessels, including the anterior/posterior perforating vessels, choroidal vessels, or posterior cerebral vessels [27, 28]. Vinuela et al reported that out of 405 total patients, the AVM could only be cured in 9.9% of them, and those lesions that could be cured were relatively small in size [13, 24]. Henkes et al have shown that endovascular embolization has succeeded in reducing the size of the nidus of AVM from 10-95% with complications only arising in 6% of 64 total patients [29]. In more recent literature, hemorrhagic complication from embolization ranges from 2% to 4.7%, resulting from arterial perforation, intranidal aneurysm rupture, or untoward venous occlusion, while mortality rates are less than approximately 1% [23, 24, 30, 31].
Embolization prior to radiosurgery
The use of endovascular embolization before radiosurgery on AVMs has the goals of decreasing the AVM size to less than three centimeters in diameter, removing aneurysms and other factors that could result in hemorrhage, and reducing symptoms caused by venous hypertension [32, 33]. There is no consensus on a particular embolic material to be used in embolization before radiosurgery [34, 35]. Reports have shown that there is delayed recanalization of AVMs after the use of polyvinyl alcohol as an embolic agent [13, 36]. Consequently, it is largely recommended to use more permanent embolic agents including polymers of cyanoacrylate, but studies have still shown a recanalization rate of 14% with these agents [34, 35]. Gobin et al have reported a study in which embolization cured only about 11% of patients with AVM, but the lesions were reduced enough to allow for radiosurgery in 76% of the patients [3, 37-40].
Palliative embolization
Endovascular treatment typically includes palliative embolization for patients who have large, inoperable cortical and subcortical AVMs or for those with seizures or progressive neurological deficit [41-43]. Palliative embolization should be employed to treat a specific feature related to an AVM, such as an associated aneurysm, or to eradicate a specific symptom. However, whether or not AVM embolization reduces hemorrhagic risk in the future is unclear. As stated before, endovascular embolization of AVMs as the sole treatment is only successful in small lesions fed by 4 or less arterial pedicles [13, 24]. Permanent occlusion of brain AVMs by endovascular embolization alone is achieved in only 10% to 30 % of patients [14-17].
| Case Reports | ▴Top |
Case 1
A 27-year-old G1P0 woman at 11.5 weeks gestation presented to the ER with post-coital sudden onset headache accompanied by numbness/tingling in left upper extremity. Her exam upon presentation was significant only for decreased sensation in her left upper extremity. Despite being gravid, head CT was indicated due to her clinical status, which showed right-sided periventricular intraparenchymal hemorrhage (IPH) with bilateral IVH (Fig. 1a). CT angiography of the head showed Spetzler-Martin Grade IV AVM located at the right lentiform nucleus and right thalamus (Fig. 1b). Her initial treatment included an external ventriculostomy drain (EVD). Follow-up head CT in the following month showed a reduction of the hemorrhage, thus not warranting an immediate ventriculoperitoneal shunt (VPS). Following an uncomplicated Cesarean delivery, the patient’s neurologic status was intact except for minor memory deficits. She decided to postpone any intervention regarding her IPH.
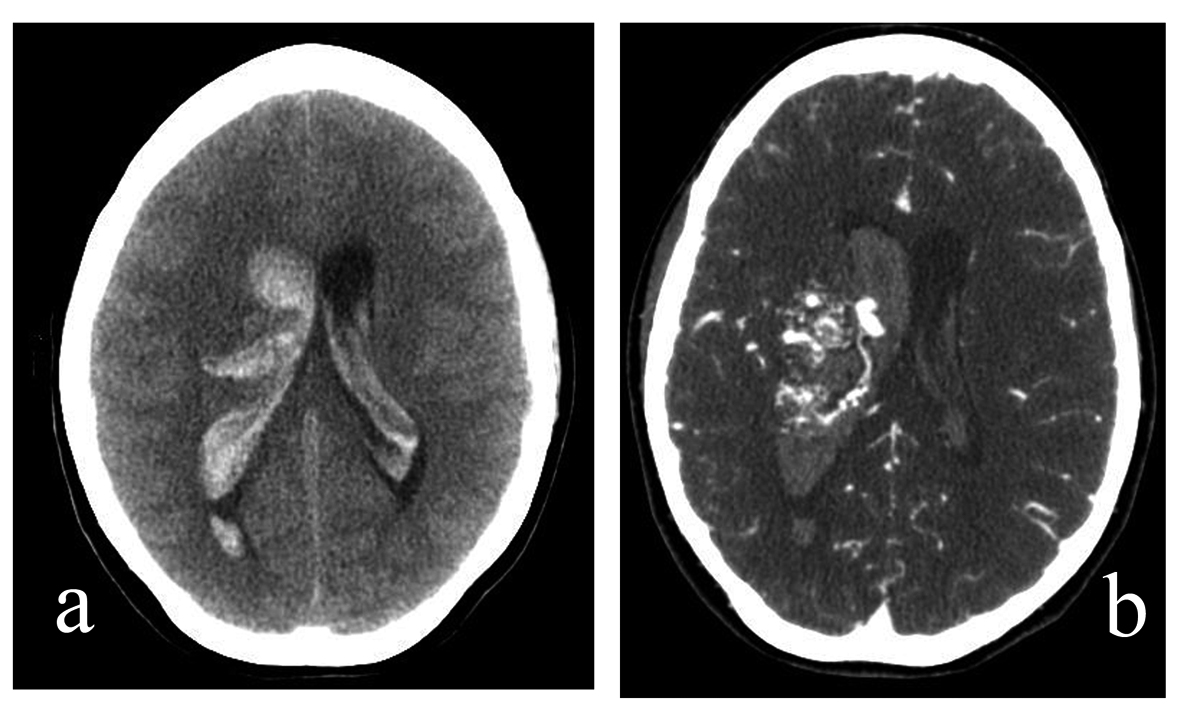 Click for large image | Figure 1. (a). A 27-year-old G1P0 woman presented to the ER at 11.5 weeks gestation with a sudden onset headache accompanied by left upper extremity numbness and tingling, which began during coitus. Non-contrast head CT, axial cut. Note right thalamic intraparenchymal hemorrhage with intraventricular hemorrhage. (b). A 27-year-old G1P0 woman presented to the ER at 11.5 weeks gestation with a sudden onset headache accompanied by left upper extremity numbness and tingling, which began during coitus. CT angiography of the head, axial cut. Note presence of AVM in right lentiform nucleus and right thalamus. |
Case 2
A 26-year-old G4P2012 woman with a history of stage 2 embolization of a left parietal AVM developed worsening of symptoms including severe migraines with concomitant vomiting, dizziness, and right-sided weakness during her pregnancy. CT angiography of the head showed a large AVM located in the left posterior parietal cortex without evidence of intracranial hemorrhage (Fig. 2). She underwent an uncomplicated Cesarean delivery at 36 weeks and resumed stage 3 embolization 9 months after her delivery. Significant improvement in her concentration was noted; however, embolization had to be postponed due to another pregnancy. During this pregnancy, she suffered left arm rigidity with occasional twitches. It was later ascertained that she was non-compliant with her phenytoin seizure prophylaxis. After delivery, she reported right visual field deficits in both eyes. She continued her embolization regimen, completing stage 6 ten months ago, and recently underwent stereotactic radiosurgery. She continues to suffer migraine headaches and is closely being followed.
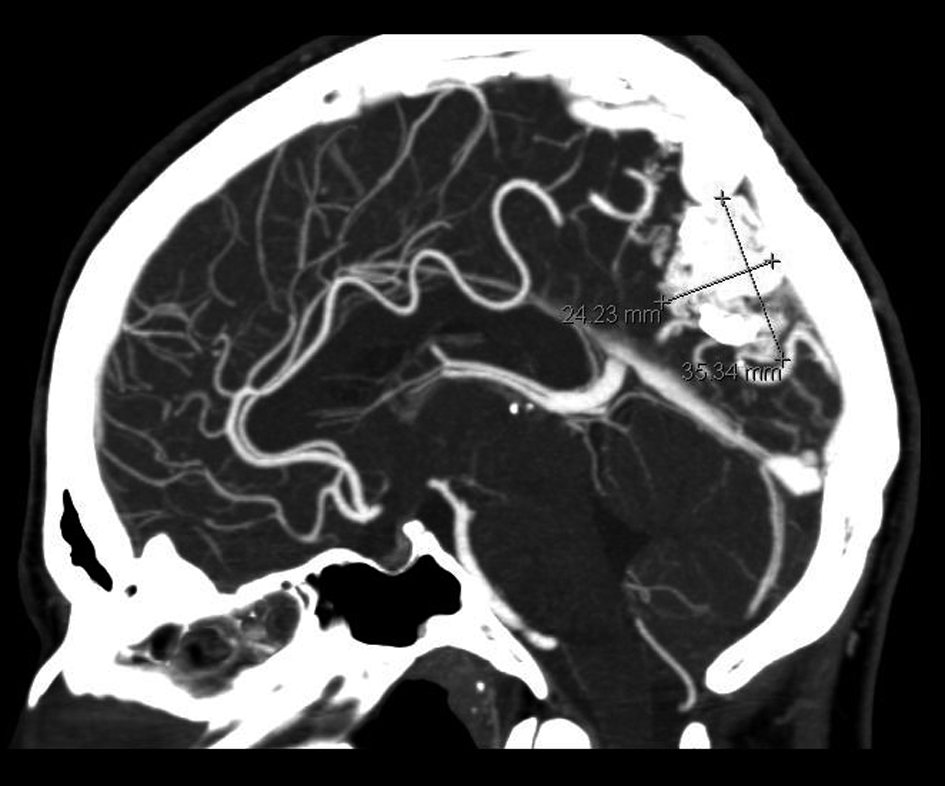 Click for large image | Figure 2. A 26-year-old G4P2012 woman with a left parietal AVM and a prior stage 2 embolization developed a worsening of symptoms, including migraines, vomiting, dizziness, and right-sided weakness, during her pregnancy. CT angiography of the head, sagittal cut. Note presence of left posterior parietal AVM with prior stage 2 embolization. |
| Discussion | ▴Top |
Cerebral AVMs in pregnant women may warrant an increased level of caution due to the potential for increased risk of hemorrhage. Given the increase in cardiac output during pregnancy and labor specifically, it is believed that the fragile AVM loses stability and becomes more susceptible to rupture [8, 44-46]. While increased risk is indefinite, these two case reports demonstrate the caution with which AVM must be treated in pregnant women. More specifically, multidisciplinary therapy would likely be useful in pregnant women with AVM due to the dynamic nature of the lesions [1]. Therapeutic strategies should be catered to individual patient profiles.
In Case 1, the patient presented with the hallmark symptoms of cerebral hemorrhage, a sudden and severe headache [1]. Thus, CT angiography was quickly determined to be the proper diagnostic measure, and after determining the severity of her lesion, initial treatment of EVD was determined to be suitable. In this case, surgical intervention was risky, given the patient’s grade of IV on the Spetzler-Martin scale [1]. This scale is utilized to grade AVMs on the basis of three traits predictive of surgical outcomes: the maximum diameter of the AVM, its location (within or outside the eloquent core), and the presence or absence of deep venous drainage [1]. Here, the pertinent question is when is the most appropriate to operate on a pregnant woman with an AVM. Indeed, the answer is uncertain and is largely dependent upon various factors, such as the patient’s age, neurological status, associated clinical risk factors, specific features of the lesion, and the neurosurgeon’s professional opinion. Some series have reported that the hemorrhage rate from AVMs in pregnancy is around 0.6% to 3.5%, which is similar to the 2% to 4% rate in non-pregnant women [10]. Therefore, since this is considered a low risk of hemorrhage, most authors suggest a conservative management of unruptured AVMs in pregnancy [10]. A more urgent and demanding method, however, is required when a pregnant woman presents with a ruptured AVM because the risk of re-bleed during the same pregnancy (27% to 30%) is much greater than the risk of re-bleed in non-pregnant women within one year of their initial bleed (6%) [10]. Moreover, rupture of an intracranial AVM during pregnancy necessitates immediate attention to preserving the mother’s life as well as the fetus’ well being. In this case, the hemorrhage had already occurred, increasing the risk of re-bleeding after an initial AVM rupture and the correlated need to eradicate the AVM [47]. Ultimately, EVD was a successful approach for the Case 1 patient. She suffered no complications and the overall outcome of the procedure was successful reduction of the hemorrhage [1, 3].
In Case 2, the patient complained of severe migraines with concomitant vomiting and dizziness. CT angiography revealed the presence of a large AVM located in the left posterior parietal cortex. However, the patient had not yet suffered a hemorrhage. Thus, an invasive procedure was deemed to be unnecessary and endovascular embolization was instead implemented; most authors suggest a conservative management of unruptured AVMs in pregnancy [10]. While the patient’s treatment was successful overall, embolization is a procedure that has been proven to be minimally curative when used in isolation; several investigators have reported that this procedure is effective in 10% of patients [3, 14-17]. Indeed, it is not clear whether AVM was cured in the patient. Following this initial treatment, the patient postponed her emoblization and eventually revealed that she had not been compliant with her phenytoin seizure prophylaxis. She suffered upper extremity rigidity and twitching, visual field deficits and recurrence of migraines. It is uncertain whether there exists a correlation the patient’s inconsistent therapy and her neurological decline. However, the positive outcome of her initial treatment cannot be ignored. Pregnant women with AVM, who have not yet suffered a hemorrhage, would be well served with endovascular embolization.
Conclusion
Improvements in endovascular therapeutic strategies provide many options for treating AVMs, including presurgical embolization, embolization prior to radiosurgery, and palliative embolization. The most effective method in the management of AVMs during pregnancy is a unique, multifaceted endovascular approach, which is tailored to the clinical condition of the individual patient. A close collaboration with a group of obstetricians, neuroradiologists, anesthesiologists, and neurosurgeons is necessary to be successful.
Disclosure
The authors have no personal financial or institutional interest in any of the drugs, material, or devices described in this article.
| References | ▴Top |
- Friedlander RM. Clinical practice. Arteriovenous malformations of the brain. N Engl J Med. 2007;356(26):2704-2712.
doi pubmed - Soderman M, Andersson T, Karlsson B, Wallace MC, Edner G. Management of patients with brain arteriovenous malformations. Eur J Radiol. 2003;46(3):195-205.
doi - Fleetwood IG, Steinberg GK. Arteriovenous malformations. Lancet. 2002;359(9309):863-873.
doi - Stapf C, Labovitz DL, Sciacca RR, Mast H, Mohr JP, Sacco RL. Incidence of adult brain arteriovenous malformation hemorrhage in a prospective population-based stroke survey. Cerebrovasc Dis. 2002;13(1):43-46.
doi pubmed - Ko NU, Johnston SC, Young WL, Singh V, Klatsky AL. Distinguishing intracerebral hemorrhages caused by arteriovenous malformations. Cerebrovasc Dis. 2003;15(3):206-209.
doi pubmed - Choi EJ, Walker EJ, Shen F, Oh SP, Arthur HM, Young WL, Su H. Minimal homozygous endothelial deletion of Eng with VEGF stimulation is sufficient to cause cerebrovascular dysplasia in the adult mouse. Cerebrovasc Dis. 2012;33(6):540-547.
doi pubmed - Mikhak B, Weinsheimer S, Pawlikowska L, Poon A, Kwok PY, Lawton MT, Chen Y, et al. Angiopoietin-like 4 (ANGPTL4) gene polymorphisms and risk of brain arteriovenous malformations. Cerebrovasc Dis. 2011;31(4):338-345.
doi pubmed - English LA, Mulvey DC. Ruptured arteriovenous malformation and subarachnoid hemorrhage during emergent cesarean delivery: a case report. AANA J. 2004;72(6):423-426.
pubmed - Jeng JS, Tang SC, Yip PK. Incidence and etiologies of stroke during pregnancy and puerperium as evidenced in Taiwanese women. Cerebrovasc Dis. 2004;18(4):290-295.
doi pubmed - Trivedi RA, Kirkpatrick PJ. Arteriovenous malformations of the cerebral circulation that rupture in pregnancy. J Obstet Gynaecol. 2003;23:484.
doi pubmed - Lanzino G, Jensen ME, Cappelletto B, Kassell NF. Arteriovenous malformations that rupture during pregnancy: a management dilemma. Acta Neurochir (Wien). 1994;126(2-4):102-106.
doi - Jermakowicz WJ, Tomycz LD, Ghiassi M, Singer RJ. Use of endovascular embolization to treat a ruptured arteriovenous malformation in a pregnant woman: a case report. J Med Case Rep. 2012;6:113.
- Ogilvy CS, Stieg PE, Awad I, Brown RD, Jr., Kondziolka D, Rosenwasser R, Young WL, et al. Recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Circulation. 2001;103(21):2644-2657.
doi pubmed - Fournier D, TerBrugge KG, Willinsky R, Lasjaunias P, Montanera W. Endovascular treatment of intracerebral arteriovenous malformations: experience in 49 cases. J Neurosurg. 1991;75(2):228-233.
doi pubmed - Merland JJ, Rufenacht D, Laurent A, Guimaraens L. Endovascular treatment with isobutyl cyano acrylate in patients with arteriovenous malformation of the brain. Indications, results and complications. Acta Radiol Suppl. 1986;369:621-622.
- Schumacher M, Horton JA. Treatment of cerebral arteriovenous malformations with PVA. Results and analysis of complications. Neuroradiology. 1991;33(2):101-105.
doi pubmed - Ter Brugge KG, Lasjaunias P, Chiu MC. Surgical neuroangiography of intracranial vascular malformations. Can J Neurol Sci. 1987;14(1):70-74.
pubmed - Jafar JJ, Davis AJ, Berenstein A, Choi IS, Kupersmith MJ. The effect of embolization with N-butyl cyanoacrylate prior to surgical resection of cerebral arteriovenous malformations. J Neurosurg. 1993;78(1):60-69.
doi pubmed - Gobin YP, Laurent A, Merienne L, Schlienger M, Aymard A, Houdart E, Casasco A, et al. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg. 1996;85(1):19-28.
doi pubmed - Taylor CL, Dutton K, Rappard G, Pride GL, Replogle R, Purdy PD, White J, et al. Complications of preoperative embolization of cerebral arteriovenous malformations. J Neurosurg. 2004;100(5):810-812.
doi pubmed - Heros RC. Embolization of arteriovenous malformations. J Neurosurg. 2004;100(5):807-809; discussion 809.
doi pubmed - Cromwell LD, Harris AB. Treatment of cerebral arteriovenous malformations: combined neurosurgical and neuroradiologic approach. AJNR Am J Neuroradiol. 1983;4(3):366-368.
pubmed - Spetzler RF, Martin NA, Carter LP, Flom RA, Raudzens PA, Wilkinson E. Surgical management of large AVM's by staged embolization and operative excision. J Neurosurg. 1987;67(1):17-28.
doi pubmed - Vinuela F, Dion JE, Duckwiler G, Martin NA, Lylyk P, Fox A, Pelz D, et al. Combined endovascular embolization and surgery in the management of cerebral arteriovenous malformations: experience with 101 cases. J Neurosurg. 1991;75(6):856-864.
doi pubmed - Yakes WF, Luethke JM, Parker SH, Stavros AT, Rak KM, Hopper KD, Dreisbach JN, et al. Ethanol embolization of vascular malformations. Radiographics. 1990;10(5):787-796.
pubmed - Purdy PD, Samson D, Batjer HH, Risser RC. Preoperative embolization of cerebral arteriovenous malformations with polyvinyl alcohol particles: experience in 51 adults. AJNR Am J Neuroradiol. 1990;11(3):501-510.
pubmed - Liebman KM, Rosenwasser RH. The hemodynamic changes measured in cerebral arterio-venous malformations following endovascular treatment. Journal of Neurovascular Disease 1997;2:112-116.
- Vinuela F, Fox AJ, Pelz D, Debrun G. Angiographic follow-up of large cerebral AVMs incompletely embolized with isobutyl-2-cyanoacrylate. AJNR Am J Neuroradiol. 1986;7(5):919-925.
pubmed - Henkes H, Nahser HC, Berg-Dammer E, Weber W, Lange S, Kuhne D. Endovascular therapy of brain AVMs prior to radiosurgery. Neurol Res. 1998;20(6):479-492.
pubmed - Purdy PD, Batjer HH, Risser RC, Samson D. Arteriovenous malformations of the brain: choosing embolic materials to enhance safety and ease of excision. J Neurosurg. 1992;77(2):217-222.
doi pubmed - Purdy PD, Batjer HH, Samson D, Risser RC, Bowman GW. Intraarterial sodium amytal administration to guide preoperative embolization of cerebral arteriovenous malformations. J Neurosurg Anesthesiol. 1991;3(2):103-106.
doi pubmed - Dawson RC, 3rd, Tarr RW, Hecht ST, Jungreis CA, Lunsford LD, Coffey R, Horton JA. Treatment of arteriovenous malformations of the brain with combined embolization and stereotactic radiosurgery: results after 1 and 2 years. AJNR Am J Neuroradiol. 1990;11(5):857-864.
pubmed - Dion JE, Mathis JM. Cranial arteriovenous malformations. The role of embolization and stereotactic surgery. Neurosurg Clin N Am. 1994;5(3):459-474.
pubmed - Fournier D, Terbrugge K, Rodesch G, Lasjaunias P. Revascularization of brain arteriovenous malformations after embolization with bucrylate. Neuroradiology. 1990;32(6):497-501.
doi pubmed - Rao VR, Mandalam KR, Gupta AK, Kumar S, Joseph S. Dissolution of isobutyl 2-cyanoacrylate on long-term follow-up. AJNR Am J Neuroradiol. 1989;10(1):135-141.
pubmed - Pollack JM. The role of stereotactic radiosurgery for central nervous system tumors. Cancer Invest. 1996;14(5):501-502.
doi pubmed - Brown RD, Jr., Wiebers DO, Forbes G, O'Fallon WM, Piepgras DG, Marsh WR, Maciunas RJ. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg. 1988;68(3):352-357.
doi pubmed - Crawford PM, West CR, Chadwick DW, Shaw MD. Arteriovenous malformations of the brain: natural history in unoperated patients. J Neurol Neurosurg Psychiatry. 1986;49(1):1-10.
doi pubmed - Graf CJ, Perret GE, Torner JC. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983;58(3):331-337.
doi pubmed - Ondra SL, Troupp H, George ED, Schwab K. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg. 1990;73(3):387-391.
doi pubmed - Berenstein A, Lasjaunias P. Surgical neurangiography. New York, NY, Springer-Verlag, 1987.
- Fox AJ, Girvin JP, Vinuela F, Drake CG. Rolandic arteriovenous malformations: improvement in limb function by IBC embolization. AJNR Am J Neuroradiol. 1985;6(4):575-582.
pubmed - Vinuela FV, Debrun GM, Fox AJ, Girvin JP, Peerless SJ. Dominant-hemisphere arteriovenous malformations: therapeutic embolization with isobutyl-2-cyanoacrylate. AJNR Am J Neuroradiol. 1983;4(4):959-966.
pubmed - Biller J, Adams HP, Jr. Cerebrovascular disorders associated with pregnancy. Am Fam Physician. 1986;33(6):125-132.
pubmed - Robinson JL, Hall CS, Sedzimir CB. Arteriovenous malformations, aneurysms, and pregnancy. J Neurosurg. 1974;41(1):63-70.
doi pubmed - Sharma SK, Herrera ER, Sidawi JE, Leveno KJ. The pregnant patient with an intracranial arteriovenous malformation. Cesarean or vaginal delivery using regional or general anesthesia? Reg Anesth. 1995;20(5):455-458.
pubmed - Itoyama Y, Uemura S, Ushio Y, Kuratsu J, Nonaka N, Wada H, Sano Y, et al. Natural course of unoperated intracranial arteriovenous malformations: study of 50 cases. J Neurosurg. 1989;71(6):805-809.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.







